Status of coexisting chronic obstructive pulmonary disease and its clinicopathological features in patients undergoing lung cancer surgery: a cross-sectional study of 3,006 cases
Introduction
Lung cancer is the most common malignancy and remains the number one killer among all kinds of cancers worldwide (1). Over the past decades, due to tobacco consumption, environmental pollution and occupational exposure, the morbidity and mortality of lung cancer have been increasing dramatically in the world and especially in China (2). Chronic obstructive pulmonary disease (COPD) as another major respiratory disease is characterized by persistent inflammation in airways, leading to airflow limitation that cannot be completely reversed and progressive decline in lung function (3). During the last decade, increasing studies have shown that lung cancer and COPD are far from irrelevant: despite shared etiological factors such as cigarette smoking and air pollution, COPD is an independent risk factor for developing lung cancer. The risk of lung cancer in patients with COPD is two to six times higher than those without COPD (4). On the other hand, nearly 50–80% of patients diagnosed with lung cancer have existed COPD (5).
In recent years, even though great advances have been made in chemoradiotherapy and targeted therapy, curative surgery is still the most effective treatment for lung cancer at early stage (6). However, when surgical treatment comes to patients with operable lung cancer who have a coexistence of COPD, things are different. Patients with COPD have pre-existing impairment of lung function, which may fail to reach the respiratory evaluation standard required in lung resection (7), thus gaining relatively lower opportunities to receive lung cancer surgery (8). And worse still, a great number of studies demonstrated that even patients with impaired lung function could go through lung cancer surgery, an increased risk for postoperative complications such as pneumonia and persistent air leak would be another problem for them (9). Patients of lung cancer with coexistence of COPD had worse postoperative overall survival than patients without COPD (10,11), which can be partially attributed to the fact that COPD might accelerate lung cancer recurrence after surgery (12,13). Considering coexistence of COPD exerts seriously adverse impacts on lung cancer surgery and surgical curative effects, it is essential to investigate the status of coexisting COPD and figure out its clinicopathological characteristics in patients undergoing lung cancer surgery.
Methods
Patients
Clinical data of patients undergoing lung cancer surgery in the department of thoracic surgery of Zhongshan Hospital of Fudan University from January 2008 to April 2014 were gathered from hospital records databases for this research. Inclusion criteria: patients with primary lung cancer which was pathologically confirmed after surgery. Exclusion criteria: patients with lung cancer diagnosed as metastasis due to primary tumors in other organs such as colorectal cancer and liver cancer; patients complicated with primary tumors in other organs simultaneously; patients who underwent lung resection for more than one time; patients who had received chemoradiotherapy before surgery; patients without complete clinical and pathological information. Finally, a total of 3,006 cases meeting the criteria above were selected for this study.
Clinicopathological parameters
Demographic and clinicopathological parameters gathered for this analysis included age, gender, smoking status, peripheral white blood cell (WBC) count, initial symptoms, tumor size measured by the largest diameter of lung cancer, histological subtype, histological differentiation, pathological TNM stage, surgical type, length of hospital stay and patient’s post-bronchodilator lung functions such as forced expiratory volume in one second (FEV1), ratio of FEV1 to predicted values (FEV1%pred), forced vital capacity (FVC), ratio of FVC to predicted values (FVC%pred), ratio of FEV1 to FVC (FEV1/FVC), ratio of residual volume to total lung capacity (RV/TLC), diffusion capacity for carbon monoxide of the lung-single breath (DLCO-SB). All lung function values were preoperative. According to Global Initiative for Chronic Obstructive Lung Disease (GOLD) standard for diagnosis and grade of COPD (14), patients with a post-bronchodilator FEV1/FVC <70% were defined as COPD (FEV1%pred ≥80% as mild-COPD; 50%≤ FEV1%pred <80% as moderate-COPD; FEV1%pred <50% as severe-COPD). Patients with FEV1/FVC ≥70% were defined as non-COPD. Clinicopathological parameters mentioned above were compared among the different groups.
Statistical analysis
Quantitative data were expressed as mean ± SD (standard deviation) or median (interquartile range). Data with normal distribution were analyzed using one-way analysis of variance or Student’s t-test. Data with abnormal distribution were analyzed using the nonparametric Mann-Whitney U test. Categorical data were expressed as percentages and analyzed using Chi-square test. Ranked data such as tumor stage were analyzed using the Mann-Whitney U test. Length of hospital stay were also analyzed using the Kaplan-Meier method with the log-rank test. All statistical analyses were performed by SPSS for Windows (Version 17.0, Chicago, IL, USA). All P values were two-sided and a difference was considered statistically significant at P<0.05.
Results
Status of coexisting COPD
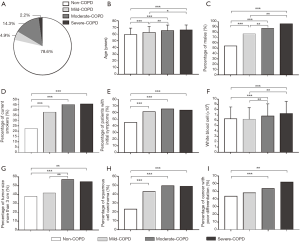
A total of 643 cases (21.4%) were complicated with COPD in 3,006 patients, of which 148 cases (4.9%) were mild COPD, 429 cases (14.3%) were moderate COPD and 66 cases (2.2%) were severe COPD (Figure 1A). Except for FVC (L), the differences of all other lung function parameters between the COPD group and the non-COPD group were statistically significant (P<0.05). FEV1, FEV1%pred, FVC%pred, FEV1/FVC and DLCO-SB in the COPD group were significantly inferior to the counterparts in the non-COPD group (P<0.05), while RV/TLC presented the opposite trend (P<0.05), as presented in Table 1.

Full table
Comparison of clinicopathological characteristics between patients with and without COPD
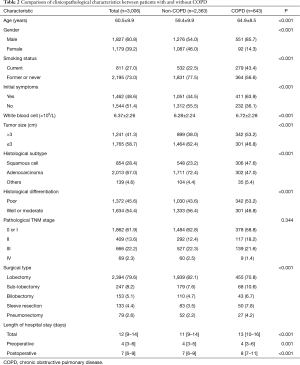
As presented in Table 2. Patients with COPD (64.9±8.5 years) were significantly older than those without COPD (59.4±9.9 years). Males (85.7% vs. 54.0%) and current smokers (43.4% vs. 22.5%) were both markedly more in the COPD group than the non-COPD group (P<0.05). Patients with COPD presented significantly more initial symptoms than patients without COPD (63.9% vs. 44.5%, P<0.05), including cough or sputum, bloody sputum or hemoptysis, chest tightness or dyspnea and chest pain (all P<0.05) (Table 3). Patients with COPD were more likely to manifest two or more symptoms compared to those without COPD (23.2% vs. 14.0%, P<0.05) (Table 3). WBC count was higher in the COPD group than the non-COPD group [(6.72±2.28 vs. 6.28±2.24) ×109/L, P<0.05].
Full table

Full table
As presented in Table 2. Patients with COPD were more likely to have bigger tumor with size more than 3 cm than patients without COPD (53.2% vs. 38.0%, P<0.05). Adenocarcinoma (72.4%) was the most common histological subtype in patients without COPD, while squamous cell carcinoma (47.6%) and adenocarcinoma (47.0%) happened similarly frequently in patients with COPD. A higher percentage of lung cancer with poor differentiation was found in the COPD group than the non-COPD group (53.2% vs. 43.6%, P<0.05). However, the difference of tumor pathological stage between the COPD group and the non-COPD group was not significant (P>0.05).
As presented in Table 2. Although lobectomy was the predominant surgical type for patients with or without COPD, sub-lobectomy and sleeve resection were more frequent in the COPD group than the non-COPD group (P<0.05). The median total length of hospital stay was significantly longer in the COPD group than the non-COPD group (13 vs. 11 days, P<0.05), so was the median postoperative length of hospital stay (8 vs. 7 days, P<0.05).
Comparison of clinicopathological characteristics among patients with different severities of COPD and without COPD
Clinicopathological characteristics were further compared among the non-COPD group, the mild-COPD group, the moderate-COPD group and the severe-COPD group. Both age and percentage of males presented significantly increasing trend with declining of lung function, as shown in Figure1B,C, respectively. Patients with COPD, no matter the grade of the severity, were all prone to be current smokers, as shown in Figure1D. The percentage of patients with initial symptoms in the four groups above were 44.5%, 60.8%, 65.3% and 62.1%, respectively. Patients with COPD, no matter its severity, presented markedly more symptoms than patients without COPD, while no significant difference when compared within the mild, moderate and severe COPD groups (P>0.05), as shown in Figure1E. WBC count in patients with mild COPD was similar to that of patients without COPD, while WBC count in patients with moderate to severe COPD started to increase significantly as compared to patients without COPD (P<0.05), but no further significantly higher as more severity of COPD, as shown in Figure1F.
Patients with moderate and severe COPD significantly had bigger tumor, size more than 3 cm, than patients without COPD or just with mild COPD, with percentage more than half, as shown in Figure1G. Squamous cell carcinoma in patients without COPD was much less than patients with COPD, while no significant difference among the COPD groups with different severities (P>0.05), as shown in Figure1H. Patients with moderate and severe COPD but not mild COPD had significantly higher percentages of cancer with poor differentiation than patients without COPD (P<0.05), as shown in Figure1I.
As shown in Figure 2A. The median total length of hospital stay in the four groups above were 11 [9–14] days, 12 (10–14.75) days, 13 [11–17] days and 13 [10–16] days, which significantly gradually increased by one day from patients without COPD to patients with mild-COPD and then to patients with moderate or severe COPD (P<0.05). And this result was also demonstrated through the nearly entire overlap of the orange curve indicating the moderate-COPD and the red curve indicating the severe-COPD, which was, however, separated from the blue curve indicating the mild-COPD and the green curve indicating the non-COPD at intervals of one day with the log-rank P<0.001, as shown in Figure 2B.
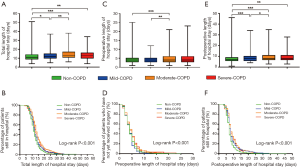
As shown in Figure 2C. The median preoperative length of hospital stay in the four groups above were 4 [3–5], 4 [3–5], 4 [3–6] and 4 [3–6] days. Only patients with moderate COPD have longer preoperative length of hospital stay than patients with mild COPD or without COPD. And this result can be also partially demonstrated through the nearly entire overlap of the orange curve indicating the moderate-COPD and the red curve indicating the severe-COPD, which was, however, partially separated from the nearly entire overlap of the blue curve indicating the mild-COPD and the green curve indicating the non-COPD with the log-rank P<0.001, as shown in Figure 2D.
As shown in Figure 2E. The median postoperative length of hospital stay in the four groups above were 7 [6–9] days, 8 [6–10] days, 8 [7–11] days and 8 [7–11] days. Much similarly to the result from total length of hospital stay, the median postoperative length of hospital stay significantly gradually increased by one day from patients without COPD to patients with mild-COPD and then to patients with moderate or severe COPD (P<0.05). And this result was also demonstrated through the nearly entire overlap of the orange curve indicating the moderate-COPD and the red curve indicating the severe-COPD, which was, however, separated from the blue curve indicating the mild-COPD and the green curve indicating the non-COPD with the log-rank P<0.001, as shown in Figure 2F.
Discussion
To date, although it has been well established that coexistence of COPD not only reduces operative chance but also increases postoperative complications and promotes cancer recurrence, which imposes seriously unfavorable impacts on prognosis of lung cancer patients after surgical resection (8-13), the status of coexisting COPD in patients with lung cancer is still substantially unnoticed by clinicians and especially by thoracic surgeons (15,16). It was reported that even for patients with coexistence of COPD, different severities of COPD carried different risks for postoperative complications and patients with different severities of COPD showed disparate survival after lung cancer surgery (17). Hence, it is worth mentioning that different severities of COPD should be grouped in clinical study. In the present study, we showed that about 21.4% of patients undergoing lung cancer surgery had coexistence of COPD, among whom moderate-COPD was the most frequent, followed by mild and severe COPD, which implied less benefits they might obtain from the surgery and more attention should be paid to this specific population.
Our study demonstrated that more male elder patients were with coexistence of COPD, which were gradually increased with the severity of COPD. Patients with coexistence of COPD, no matter how severe the COPD was, were all prone to be current smokers. Zhai (18) and colleagues reported that cigarette smoking was the most important risk factor for developing lung cancer combined with COPD, especially in females and those with histology of squamous cell carcinoma.
Our study also found that patients with coexistence of COPD were more vulnerable to respiratory initial symptoms responsible for a clinical visit. In addition to smoking, we hypothesized reasons for this phenomenon might be: patients with COPD preexist narrowed airways and mucus hypersecretion (19), subsequent neoplasm formation in airways could be easily to trigger irritating cough or sputum; secondly, as shown in our study, lung cancer with COPD was often bigger in size and poorer in differentiation, which might grow faster and more easily completely obstruct airways to cause more frequent shortness of breath, chest tightness or dyspnea; thirdly, lung cancer with COPD, which is highly malignant and in a rapid growth condition, may be easier to generate tumor necrosis or invasion to surrounding micro-vessels, which resulted in more frequent bloody sputum or hemoptysis.
COPD is not only a respiratory inflammatory disease but also a systemic inflammatory disorder (20). WBC count, a biomarker measuring the systemic inflammatory level, is closely related to the severity of COPD (21). And it has been shown that the preoperative WBC count was an independent risk factor for postoperative cardiopulmonary complications after lobectomy for lung cancer (OR =1.451, 95% CI: 1.212–1.736) (22). A persistent high level of systemic inflammation may increase the risk for postoperative pneumonia in patients undergoing lung cancer surgery (23). Our study showed that WBC count in patients with COPD was significantly higher than those without COPD, consistent with the previous studies indicating that coexistence of COPD would increase postoperative complications after lung cancer surgery (9,24). Furthermore, we found that only patients with moderate or severe COPD but not mild COPD had a significantly higher WBC compared to those without COPD, consistent with the previous study indicating that only moderate or severe COPD was associated with increased postoperative pulmonary complications (11).
In the present study, it was obviously demonstrated that the total length of hospital stay was gradually increased at intervals of one day with the aggravating severity of COPD, which can be mainly attributed to the extended postoperative length of hospital stay. To some extent, the poorer lung function shown in our study and the more postoperative complications of patients with coexistence of COPD shown by others (9,11) may be responsible for this prolonged length of hospital stay. At last, from what has been discussed above, we easily found that all comparisons of clinicopathological parameters between patients with moderate COPD and patients with severe COPD were not statistically different, indicating that they indeed owned extremely similar clinicopathological characteristics. From this point, it is possible that we could gather up the moderate COPD and the severe COPD but separate the mild COPD for analysis in future clinical study.
In a word, the clinicopathological features of lung cancer combined with COPD are significantly distinguished from the counterparts of lung cancer alone, thoracic surgeons should attach great importance to coexistence of COPD and be familiar with its clinicopathological features in patients undergoing lung cancer surgery. With the help of pulmonologists, physicians who are really professional in COPD management (25), the status of coexisting COPD should be accurately diagnosed and adequately treated during the perioperative period of lung cancer.
Acknowledgements
Funding: This work was supported by the Sub-project (2017YFC1310602) of National Key Special Project of Ministry of Science and Technology of China (2017YFC1310600), Research Foundation of Shanghai Municipal Commission of Health and Family Planning (201540078), National Natural Science Foundation of China (81100048).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics committee of Zhongshan Hospital, Fudan University.
References
- Malhotra J, Malvezzi M, Negri E, et al. Risk factors for lung cancer worldwide. Eur Respir J 2016;48:889-902. [Crossref] [PubMed]
- She J, Yang P, Hong Q, et al. Lung cancer in China: challenges and interventions. Chest 2013;143:1117-26. [Crossref] [PubMed]
- Zhou Y, Zhong NS, Li X, et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med 2017;377:923-35. [Crossref] [PubMed]
- Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med 2013;1:73-83. [Crossref] [PubMed]
- Sekine Y, Hata A, Koh E, et al. Lung carcinogenesis from chronic obstructive pulmonary disease: characteristics of lung cancer from COPD and contribution of signal transducers and lung stem cells in the inflammatory microenvironment. Gen Thorac Cardiovasc Surg 2014;62:415-21. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Spyratos D, Zarogoulidis P, Porpodis K, et al. Preoperative evaluation for lung cancer resection. J Thorac Dis 2014;6:S162-6. [PubMed]
- Hashimoto N, Matsuzaki A, Okada Y, et al. Clinical impact of prevalence and severity of COPD on the decision-making process for therapeutic management of lung cancer patients. BMC Pulm Med 2014;14:14. [Crossref] [PubMed]
- Kim ES, Kim YT, Kang CH, et al. Prevalence of and risk factors for postoperative pulmonary complications after lung cancer surgery in patients with early-stage COPD. Int J Chron Obstruct Pulmon Dis 2016;11:1317-26. [Crossref] [PubMed]
- Zhai R, Yu X, Shafer A, et al. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest 2014;145:346-53. [Crossref] [PubMed]
- Yoshida Y, Kage H, Murakawa T, et al. Worse Prognosis for Stage IA lung cancer patients with smoking history and more severe chronic obstructive pulmonary disease. Ann Thorac Cardiovasc Surg 2015;21:194-200. [Crossref] [PubMed]
- Qiang G, Liang C, Xiao F, et al. Impact of chronic obstructive pulmonary disease on postoperative recurrence in patients with resected non-small-cell lung cancer. Int J Chron Obstruct Pulmon Dis 2015;11:43-9. [PubMed]
- Kuo CH, Wu CY, Lee KY, et al. Chronic obstructive pulmonary disease in stage I non-small cell lung cancer that underwent anatomic resection: the role of a recurrence promoter. COPD 2014;11:407-13. [Crossref] [PubMed]
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD Executive Summary. Am J Respir Crit Care Med 2017;195:557-82. [Crossref] [PubMed]
- Zhang R, Tan X, Chen Q, et al. Investigation of lung cancer patients complicated with chronic obstructive pulmonary disease in thoracic surgical department. Zhongguo Fei Ai Za Zhi 2017;20:163-7. [PubMed]
- Zhang J, Zhou JB, Lin XF, et al. Prevalence of undiagnosed and undertreated chronic obstructive pulmonary disease in lung cancer population. Respirology 2013;18:297-302. [Crossref] [PubMed]
- Sekine Y, Suzuki H, Yamada Y, et al. Severity of chronic obstructive pulmonary disease and its relationship to lung cancer prognosis after surgical resection. Thorac Cardiovasc Surg 2013;61:124-30. [PubMed]
- Zhai R, Yu X, Wei Y, et al. Smoking and smoking cessation in relation to the development of co-existing non-small cell lung cancer with chronic obstructive pulmonary disease. Int J Cancer 2014;134:961-70. [Crossref] [PubMed]
- Martin C, Frija-Masson J, Burgel PR. Targeting mucus hypersecretion: new therapeutic opportunities for COPD? Drugs 2014;74:1073-89. [Crossref] [PubMed]
- MacNee W. Systemic inflammatory biomarkers and co-morbidities of chronic obstructive pulmonary disease. Ann Med 2013;45:291-300. [Crossref] [PubMed]
- Koo HK, Kang HK, Song P, et al. Systemic white blood cell count as a biomarker associated with severity of chronic obstructive lung disease. Tuberc Respir Dis (Seoul) 2017;80:304-10. [Crossref] [PubMed]
- Lai Y, Su J, Wang M, et al. Classification and Risk-factor Analysis of Postoperative Cardio-pulmonary Complications after Lobectomy in Patients with Stage I non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2016;19:286-92. [PubMed]
- Nojiri T, Inoue M, Maeda H, et al. Low-dose human atrial natriuretic peptide for the prevention of postoperative cardiopulmonary complications in chronic obstructive pulmonary disease patients undergoing lung cancer surgery. Eur J Cardiothorac Surg 2013;44:98-103. [Crossref] [PubMed]
- Lugg ST, Agostini PJ, Tikka T, et al. Long-term impact of developing a postoperative pulmonary complication after lung surgery. Thorax 2016;71:171-6. [Crossref] [PubMed]
- Deepak JA, Ng X, Feliciano J, et al. Pulmonologist involvement, stage-specific treatment, and survival in adults with non-small cell lung cancer and chronic obstructive pulmonary disease. Ann Am Thorac Soc 2015;12:742-51. [Crossref] [PubMed]


