Novel method of noninvasive ventilation supported therapeutic lavage in pulmonary alveolar proteinosis proves to relieve dyspnea, normalize pulmonary function test results and recover exercise capacity: a short communication
Introduction
PAP is an extremely rare disease with a prevalence of 1–9/1,000,000, characterized by alveolar accumulation of a lipoproteinaceous material which positively stains with periodic acid-Schiff (PAS). Uneven alveolar employment results in ventilation/perfusion (V/Q) mismatch, lung function deterioration and hypoxemia. Its etiology is either intrinsic, resulting from mutation in surfactant proteins or granulocyte macrophage-colony stimulating factor (GM-CSF) receptor genes, or extrinsic, either secondary to inhalation of toxic substances and hematological malignancies or autoimmune, with alveolar macrophages’ activity blocked by anti-GM-CSF antibodies. The last one is the most frequent variant, which represents up to 90% of all PAP cases.
Albeit non-specific, a typical finding in high-resolution computed tomography (HRCT) is a ‘‘crazy paving’’ pattern. Diagnosis is most often established upon bronchoalveolar lavage (BAL) findings (1) combined with HRTC warrants diagnosis in about 2/3 of cases, whereas in about 1/3 of cases surgical lung biopsy is required to make a firm diagnosis.
Since the publication by Ramirez et al. in 1963, whole lung lavage (WLL) under general anesthesia with a double-lumen endobronchial intubation has remained the standard treatment for PAP (2). This method provides long-lasting beneficial effects. It is usually performed unilaterally, with the patient in a supine position. The lavaged lung is filled up to functional residual capacity (FRC), and cycles of saline instillation and subsequent drainage are repeated, until clear effluent is obtained. Taking into account WLL methodology, it facilitates severe hypoxemia by elicitation of extensive V/Q mismatch due to shunt through the treated lung and respiration conducted by the contralateral lung, which interstitial tissue is often heavily affected by present disease. This mechanism sometimes creates a need for extracorporeal membrane oxygenation ECMO support (3). Moreover, uneven alveolar distribution in the lavaged lung may promote post procedure pulmonary edema which is probably responsible for the demand for prolonged ventilation and oxygen supplementation (4,5). Obviously, due to low incidence of the disease, no gold standard WLL protocols have been developed as yet.
The major indications for WLL in centers caring for PAP patients worldwide include radiographic progression, lung function deterioration and hypoxemia (4).
WLL, although considered as reasonably safe and efficient if performed in well-experienced specialized centers, has serious limitations, which include post procedure complications, the most common being fever, hypoxemia, wheezing, pneumonia and saline leakage due to malposition of the endotracheal tube (4,6). Also pneumothorax, pleural effusion, cardiac arrest (4) and one case of WLL-associated death (7) were reported. There is a possibility that mortality rate is under reported.
Hypoxemia usually occurs during the drainage phase, because blood is again redirected from the ventilated lung to the contralateral lavaged one and a decrease in airway pressure occurs, leading to perfusion of the lung undergoing BAL and thus a fall in partial oxygen pressure PaO2 (4).
Last but not least, WLL takes up to 5–10 hours of operational time and requires presence of many qualified healthcare professionals, especially when ECMO support is needed. Therefore there is a need to develop new treatment modifications which do not entail such health risks and are simultaneously cost-effective and resources-saving.
Methods and results
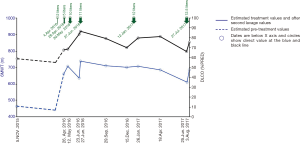
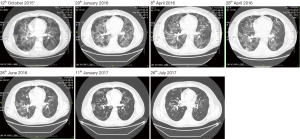
In our communication, detailed method development and its critical points are presented on an example of a 35-year-old aluminum welder with a history of environmental exposure to canaries, an ex-smoker (5 years’ history of tobacco exposure, 3 years post cessation), who was referred to our ward due to progressive exertional dyspnea. Pre-hospitalization spiral tomography suggested interstitial pneumonia, whereas bronchofiberoscopy revealed normal airway anatomy with abundant milky secretions. BAL showed a low cell count (8.7×106) with lymphocyte predominance (65%) and CD4/CD8 of 2.73. Pulmonary function tests (PFTs) revealed restriction with significant carbon monoxide diffusing capacity (DLCO) impairment (Figure 1). A 6-minute walking test (6MWT) distance was decreased and significant exertional hypoxemia was present (SaO2 86%) (8). In January, 2016 high resolution computer tomography (HRCT) showed confluent bilateral areas of ground glass opacities with crazy paving pattern (Figure 2).
Due to progressive dyspnoea, history of overlapping environmental exposure to canaries and ineffective immunosuppression with prednisone (50 mg/day) and azathioprine (150 mg/day), the patient was referred for wedge lung resection, which confirmed PAP. The patient was advised to undergo standard WLL, which he would not consent to, therefore other eligible treatment methods were considered.
After a case conference it was decided to attempt selective TLL. As TLL is more effective if larger fluid volumes are instilled and the fact that the patient presented exertional hypoxemia, it was decided to perform NIV-supported BAL. NIV-supported TLL was performed under mild sedation (10 mg midazolam, 10 mg morphine and 50 mg tramadol administered in fractionated doses). Xylocaine was administered periodically for local airway analgesia. NIV settings were adjusted based on monitored ventilation parameters (calculated to achieve 6–10 mL/kg/ideal body weight IBW), chest wall movement, and abdominal extension (Table 1). 37 °C 0.9% NaCl was applied and suctioned in portions of 60 mL/syringe. To avoid hypoxemia, TLL was performed bilaterally in most affected segments 4 & 5. Although no complications were observed, ventilation was suboptimal due to excessive leakage through open mouth (Table 1). The patient’s clinical condition slightly improved and he was advised to change profession. The procedure was performed by a single pulmonologist and two nurses trained in bronchoscopy; sedation and ventilation techniques, whereas a junior doctor was put in charge of vibration techniques lavage volume measurement and sample collection.
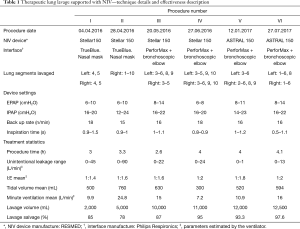
Full table
Second and all subsequent TLLs were performed with anesthesiologist assistance. Sedation was inducted with: propofol (0.5–1.0 mg/kg−1), fentanyl (1 µg/kg−1), midazolam (0.01–0.03mg/kg−1) and maintained with fractionated doses of propofol (0.1–0.3 mg/kg−1/3–5 minutes), fentanyl (1 µg/kg/hour), and midazolam (10 µg/kg/hour). During the procedure, head tilt/chin lift maneuver was performed. Based on HRCT predominance, total right lung lavage with administration of 5,000 mL of warm saline was performed. Treatment tolerance and effectiveness improved; however, ventilation was still impaired by leakage. Detailed procedure decryption is summarized in Table 1.
The third TLL during which 10,000 mL of fluid were administered was performed with the use of a dedicated full face mask with bronchoscopic elbow to decrease leakage present during two first procedures. Portions of saline (500–2,000 mL) were administered to the most involved segments, with administered volumes based on the haziness of milkish aliquot and patient tolerance (Table 1). The patient was clinically stable through the entire procedure.
After the fourth procedure, during which 11,000 mL of fluid were administered, mild post-procedure hypoxemia (SaO2: 90%) was observed, probably due to early NIV discontinuation. Hypoxemia resolved shortly after NIV reapplication through a standard oro-nasal mask. The patient became asymptomatic with DLCO exceeding 80% of predicted value (PV), 6MWT >700 m without exertional desaturations, and significant HRCT improvement (Figure 1,2). The patient was advised to return quarterly for routine check-up or earlier in case of symptoms’ relapse. Exercise induced dyspnea and exercise capacity impairment was reported six month latter and was accompanied by a decrease in DLCO.
The fifth TLL was uncomplicated with 12,000 mL of saline administered. As previously, the most affected segments were chosen for lavage (500–2,000 mL/segment). The procedure resulted in significant DLCO restoration. The patient was discharged asymptomatic. Disease stabilization lasted for 6 months despite persisting occupational exposure.
The sixth TLL was performed according to the above-mentioned ‘protocol’. Lavage was applied to the most affected segments, although HRCT generally revealed significant lesion resolution compared to earlier assessments. To avoid hypoxemia, NIV was maintained for several hours after BAL. Treatment was found to be safe and effective in terms of significant dyspnea decrease, DLCO, and 6MWT improvement.
During all procedures, approximately 80% of saline was directly suctioned and the bronchial residues from other segments were suctioned successively. Spontaneous over time (S/T) mode with low triggering was used with oxygen supplementation (10–15 L/min) resulting in fraction of inspired oxygen FiO2 of 0.31 (0.21–0.7) throughout all procedures. Detailed TLL characteristics and treatment results are presented in Figures 1,2 and Table 1. In order to increase alveolar clearance manual chest vibration techniques were used. Starting from the second TLL, all procedures were performed on average by six health care professionals: pulmonologist performing BAL and responsible for NIV adjustments, anesthesiologist with dedicated nurse responsible for patient sedation and monitoring, two nurses or nurse and physician responsible for syringes preparation, BAL collection, and physiotherapist responsible for chest-maneuvers.
Discussion
To the best of our knowledge, this is the first description of NIV as an innovative ventilation support technique which enables safe and effective TLL in PAP treatment (4). We have shown that NIV provides effective ventilation without desaturation, even when excessive lavage volumes are used. Severe hypoxemia is the most dangerous WLL complication (4,9). Based on the disease’s diverse distribution, selective high volume lavage of the most affected segments in both lungs during one procedure provides long-term results and thanks to NIV support prevents observed at WLL: severe ventilation/perfusion mismatch, volume overload, intubation and mechanical ventilation-related complications, and has a potential to reduce procedure-related costs (4).
Since the invention by Jose Ramirez-Rivera WLL under general anesthesia with a double-lumen endobronchial intubation is the world-wide standard PAP treatment (2,4,5). To increase WLL’s safety, it is usually performed in one lung during a single procedure (10). During WLL development, 3 L of saline/procedure was administered (11); which is comparable to our first attempt. Our method, due to used positive end-expiratory pressure (PEEP), stabilizes alveolar space, decreases the risk of hypoxemia, and increases the amount of recovered saline enabling selective instillation of 12.5 L of warmed saline to the most affected segments of both lungs, similar to that used in WLL (mean 15.4; range 5–40 L) (6).
During NIV-supported TLL, larger lung volumes (both lungs with exception of one segment) are simultaneously ventilated, which helps V/Q mismatch and avoid hypoxemia. Selective lavage of the most affected segments decreases the risk of post procedure pulmonary oedema and the need for prolonged ventilation. In contrast to our procedure, during WLL the lavaged lung is filled up with saline up to FRC, excessive PEEP in the ventilated lung may cause blood shunting to the treated side, which in turn contributes to serious ventilation/perfusion mismatch and hypoxemia (12).
In comparison with the aforementioned WLL-related complications (6), in our case, only mild fever and post-procedural transient hypoxemia, and headache were observed; resolving with transient post-procedural NIV application via oro-nasal mask until full conscious ness was regained.
Our method eliminates the risk of intubation-related complications (13,14). Although anesthesia was performed using short-acting drugs (15), the patient was stable during the 4-hour long procedures with no complications observed. Application of NIV enabled excessive lavage of the most affected segments based on HRCTs (Figure 2, Table 1), potentially increasing effectiveness and decreasing the risk of systemic fluid overload, as in affected segments, decreased fluid transfer from the alveoli to the vasculature may be probably expected, especially in patients with geographic distribution. In our procedure, selected segments are washed until demonstrating clear effluent or the appearance of cough, indicating a need for interim suctioning and endobronchial re-administration of 1% xylocaine. In centers performing bilateral sequential lung lavage with gravitational saline application, WLL may take 5 to 10 hours with invasive ventilation often continued post-procedure (16). Our procedure, takes approximately 4 hours. Furthermore, due to small volumes of instilled fluid, the risk of barotrauma-induced hydrothorax appears theoretical (17). Thanks to fractionated saline injections turbulent flow is generated facilitating probably increased alveolar washout.
Currently ECMO is a first-choice WLL support for severely hypoxemic patients, as WLL may aggravate hypoxemia due to blood shunting through the lavaged lung (18). Our NIV supported TLL with constant ventilation of both lungs with the except of the currently lavaged segment, creates potential for increased safety and effectiveness of PAP treatment (19) without ECMO support, reducing not only the risk of ECMO related complications, but also procedure-related costs (20,21). However, this requires prospective confirmation.
In patients with severe hypoxemia selective lobar lavage (22) or segmental lavage also referred to as “prewash” is sometimes performed with 1,600–2,600 mL to increase PaO2 and facilitate standard WLL (16). During our procedure larger fluid volumes were instilled. Therefore, our NIV supported multi-segmental lavage may be considered as more effective alternative to “prewash” in severely hypoxemic patients.
Our single case-report shows18 months long clinical stability (Figure 1). Due to absence of randomized controlled trials, it is hard to determine our treatment’s durability, especially in a patient with ongoing environmental exposure, however, WLL is often repeated to obtain clinical stability (23). Despite no calculations, our procedure appears more cost-effective compared to standard WLL as it requires less resources overall (4).
In most centers, exertional dyspnea, decreased DLCO, and 6MWT are used for treatment initiation and monitoring (4,5,24). Based on our practice, we suggest TLL when: (I) DLCO is <80% PV; (II) 6MWT values are <80% of patient PV (8) or (III) exertional hypoxemia is present (Figure 1). Decrease in DLCO observed after the third procedure may be explained by TLLs performed too frequently. This phenomenon was not seen when the time between procedures exceeded one month (Figure 1).
NIV may be considered an innovative method of ventilatory support, enabling safe, effective, and resource-saving treatment of PAP patients. Larger multicenter studies are necessary to establish a new standard of PAP treatment or to identify patients for whom NIV supported TLL may be most beneficial. We declare our readiness to start and run such collaboration. Furthermore, we assume that our method may probably be useful in patients with uneven distribution of ground glass opacities. We are also looking forward to incorporating our method into pediatrics, as our noninvasive approach may be beneficial in patients requiring frequently repeated procedures (25), or when double lumen cuffed intubation is difficult (26).
Conclusions
To conclude, TLL performed with the support of NIV seems to be a novel method of PAP treatment. Further multicenter studies are required to select patients for whom NIV supported TLL will be of greatest benefit.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Borie R, Danel C, Debray MP, et al. Pulmonary alveolar proteinosis. Eur Respir Rev 2011;20:98-107. [Crossref] [PubMed]
- Ramirez J, Nyka W, McLaughlin J. Pulmonary alveolar proteinosis: diagnostic techniques and observations. N Engl J Med 1963;268:165-71. [Crossref] [PubMed]
- Noirez L, Koutsokera A, Pantet O, et al. A 3-step therapeutic strategy for severe alveolar proteinosis. Ann Thorac Surg 2015;99:1456-8. [Crossref] [PubMed]
- Awab A, Khan MS, Youness HA. Whole lung lavage-technical details, challenges and management of complications. J Thorac Dis 2017;9:1697-706. [Crossref] [PubMed]
- Beccaria M, Luisetti M, Rodi G, et al. Long-term durable benefit after whole lung lavage in pulmonary alveolar proteinosis. Eur Respir J 2004;23:526-31. [Crossref] [PubMed]
- Campo I, Luisetti M, Griese M, et al. Whole lung lavage therapy for pulmonary alveolar proteinosis: a global survey of current practices and procedures. Orphanet J Rare Dis 2016;11:115. [Crossref] [PubMed]
- Bhagwat AG, Wentworth P, Conen PE. Observations on the relationship of desquamative interstitial pneumonia and pulmonary alveolar proteinosis in childhood: a pathologic and experimental study. Chest 1970;58:326-32. [Crossref] [PubMed]
- Przybyłowski T, Tomalak W, Siergiejko Z, et al. Polish Respiratory Society guidelines for the methodology and interpretation of the 6 minute walk test (6MWT). Pneumonol Alergol Pol 2015;83:283-97. [Crossref] [PubMed]
- Prakash UB, Barham SS, Carpenter HA, et al. Pulmonary alveolar phospholipoproteinosis: experience with 34 cases and a review. Mayo Clin Proc 1987;62:499-518. [Crossref] [PubMed]
- Claypool WD, Rogers RM, Matuschak GM. Update on the clinical diagnosis, management, and pathogenesis of pulmonary alveolar proteinosis (phospholipidosis). Chest 1984;85:550-8. [Crossref] [PubMed]
- Ramirez J, Kieffer RF Jr, Ball WC Jr. Bronchopulmonary lavage in man. Ann Intern Med 1965;63:819-28. [Crossref] [PubMed]
- Julien T, Caudine M, Barlet H, et al. Effect of positive end expiratory pressure on arterial oxygenation during bronchoalveolar lavage for proteinosis. Ann Fr Anesth Reanim 1986;5:173-6. [Crossref] [PubMed]
- Benumof JL, Partridge BL, Salvatierra C, et al. Margin of safety in positioning modern double-lumen endotracheal tubes. Anesthesiology 1987;67:729-38. [Crossref] [PubMed]
- Shah SB, Bhargava AK, Hariharan U, et al. A Randomized Clinical Trial Comparing the Standard Mcintosh Laryngoscope and the C-Mac D blade Video laryngoscope™for Double Lumen Tube Insertion for One Lung Ventilation in Onco surgical Patients. Indian J Anaesth 2016;60:312-8. [Crossref] [PubMed]
- Nandkumar S, Desai M, Butani M, et al. Pulmonary Alveolar Proteinosis with Respiratory Failure-Anaesthetic Management of Whole Lung Lavage. Indian J Anaesth 2009;53:362-6. [PubMed]
- Firat ND, Cıledağ A, Kabalak PA, et al. Pulmonary alveolar proteinosis and successful therapy with combined lavage procedures: Case reports. Exp Ther Med 2011;2:569-73. [Crossref] [PubMed]
- Kariman K, Kylstra JA, Spock A. Pulmonary alveolar proteinosis: prospective clinical experience in 23 patients for 15 years. Lung 1984;162:223-31. [Crossref] [PubMed]
- Cohen ES, Elpern E, Silver MR. Pulmonary alveolar proteinosis causing severe hypoxemic respiratory failure treated with sequential whole-lung lavage utilizing veno-venous extracorporeal membrane oxygenation: a case report and review. Chest 2001;120:1024-6. [Crossref] [PubMed]
- Bingisser R, Kaplan V, Zollinger A, et al. Whole-lung lavage in alveolar proteinosis by a modified lavage technique. Chest 1998;113:1718-9. [Crossref] [PubMed]
- Zwischenberger JB, Pitcher HT. Extracorporeal Membrane Oxygenation Management: Techniques to Liberate from Extracorporeal Membrane Oxygenation andManage Post-Intensive Care Unit Issues. Crit Care Clin 2017;33:843-53. [Crossref] [PubMed]
- Zangrillo A, Landoni G, Biondi-Zoccai G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc 2013;15:172-8. [PubMed]
- Cheng SL, Chang HT, Lau HP, et al. Pulmonary alveolar proteinosis: treatment by bronchofiberscopic lobar lavage. Chest 2002;122:1480-5. [Crossref] [PubMed]
- Shah PL, Hansell D, Lawson PR, et al. Pulmonary alveolar proteinosis: clinical aspects and current concepts on pathogenesis. Thorax 2000;55:67-77. [Crossref] [PubMed]
- Gay P, Wallaert B, Nowak S, et al. Efficacy of Whole-Lung Lavage in Pulmonary Alveolar Proteinosis: A Multicenter International Study of GELF. Respiration 2017;93:198-206. [Crossref] [PubMed]
- Griese M, Ripper J, Sibbersen A, et al. Long-term follow up and treatment of congenital alveolar proteinosis. BMC Pediatr 2011;11:72. [Crossref] [PubMed]
- Wilson CA, Wilmshurst SL, Black AE. Anesthetic techniques to facilitate lung lavage for pulmonary alveolar proteinosis in children-new airway techniques and a review of the literature. Paediatr Anaesth 2015;25:546-53. [Crossref] [PubMed]


