Prognostic factors of patients with pathologic stage I lung adenocarcinoma
Introduction
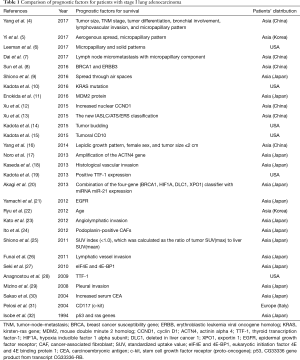
Lung cancer remains one of the most common causes of cancer-related deaths worldwide. Increasingly more research is aimed at investigating the clinicopathologic parameters or gene mutations involved in the survival of patients with lung cancer. Although the 5-year survival rate of stage I non-small cell lung cancer (NSCLC) is between 68% and 92% (stage IA1 to IB) (1), there is still much scope for progress. In our previous study (2), we reviewed the predictors of postoperative recurrence in NSCLC and postulated that those results may help optimize patient selection for specified surveillance guidelines and personalized adjuvant therapies to prevent potential occult micrometastases. In Taiwan, lung adenocarcinoma is the most common histologic subtype (12,648/21,536, 58.73%) that had become more prevalent among non-smoking women (3). Moreover, predictors of postoperative outcomes in lung adenocarcinoma have been a popular investigatory target in recent years. Therefore, we used the search engine PubMed and reviewed papers about the risk factors of postoperative outcomes in patients with stage I lung adenocarcinoma (Table 1).
Full table
Risk factors of postoperative outcomes in pathologic stage I lung adenocarcinoma
We utilized key words such as “predictors” or “risk factors” and “stage I lung adenocarcinoma” in PubMed. Twenty-nine papers were found published between 1994 and 2017. Among these, 21 (72.41%) papers were published in the last 5 years and the patient populations of 22 studies were Asian. Therefore, an increasing number of patients with early-stage lung adenocarcinoma have been treated in the recent decades and therapeutic outcomes are still being investigated. The studies listed in Table 1 provide a broad overview of the geographical distribution and high prevalence of lung adenocarcinoma in Asia.
Micropapillary components were thought as predictors of poor outcomes in patients with stage I lung adenocarcinoma in 4 studies (4-7). They have been proven to reflect an aggressive subtype of lung adenocarcinomas with poor prognosis (33-36). De Oliveira Duarte Achcar et al. (37) concluded that K-ras, EGFR, and BRAF mutations occur at increased frequencies in lung adenocarcinomas showing greater than 75% micropapillary growth. There is a close relationship between histology appearances and gene mutations. Other predictors for pathologic stage I lung adenocarcinoma are clinicopathologic parameters, such as tumor size (4,16), TNM stage (4,13), aerogenous spread (5,9), tumor differentiation (4), bronchial involvement (4), lymphovascular invasion (4,18,23,26), positive thyroid transcription factor-1 (TTF-1) expression (19,28), lepidic growth pattern (16), sex (16), age (22), tumor budding (14), pleural invasion (29), carcinoembryonic antigen levels (30), and standardized uptake value (SUV) index (<1.0) (25). Epigenetic factors or gene mutations involved with surgical outcomes of patients with stage I lung adenocarcinoma include BRCAl (8,20) and ERBB3 (8), K-ras mutation (10,32), HIF1A (20), DLC1 (20), XPO1 (20), MDM2 protein (11), increased nuclear CCND1 (12), tumoral CD10 (15), ACTN4 (17), EGFR (21), Podoplanin-positive CAFs (24), eukaryotic initiation factor 4E and 4E binding protein 1 (eIF4E and 4E-BP1) (27), CD117 (c-kit) (31), and p53 expression (32).
Conclusions
The studies identified in this review concluded many different factors affecting surgical outcomes of patients with stage I lung adenocarcinoma. It is difficult to clarify why these predictors showed different results in various studies. Although there are too many confounding factors, such as patient selection criteria, surgical techniques, or other comorbidities, these pathways could be novel therapeutic strategies for lung adenocarcinoma and these predictors could help in selecting high-risk patients with stage I lung adenocarcinoma for adjuvant therapy, such as target therapy, chemotherapy, radiotherapy, or immunotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Chen YY, Huang TW, Chang H, et al. Optimal delivery of follow-up care following pulmonary lobectomy for lung cancer. Lung Cancer (Auckl) 2016;7:29-34. [PubMed]
- Hsu CH, Tseng CH, Chiang CJ, et al. Characteristics of young lung cancer: Analysis of Taiwan's nationwide lung cancer registry focusing on epidermal growth factor receptor mutation and smoking status. Oncotarget 2016;7:46628-35. [Crossref] [PubMed]
- Yang Y, Mao Y, Yang L, et al. Prognostic factors in curatively resected pathological stage I lung adenocarcinoma. J Thorac Dis 2017;9:5267-77. [Crossref] [PubMed]
- Yi E, Bae MK, Cho S, et al. Pathological prognostic factors of recurrence in early stage lung adenocarcinoma. ANZ J Surg 2018;88:327-31. [Crossref] [PubMed]
- Leeman JE, Rimner A, Montecalvo J, et al. Histologic Subtype in Core Lung Biopsies of Early-Stage Lung Adenocarcinoma is a Prognostic Factor for Treatment Response and Failure Patterns After Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys 2017;97:138-45. [Crossref] [PubMed]
- Dai C, Xie H, Kadeer X, et al. Relationship of Lymph Node Micrometastasis and Micropapillary Component and Their Joint Influence on Prognosis of Patients With Stage I Lung Adenocarcinoma. Am J Surg Pathol 2017;41:1212-20. [Crossref] [PubMed]
- Sun Y, Hou L, Yang Y, et al. Two-gene signature improves the discriminatory power of IASLC/ATS/ERS classification to predict the survival of patients with early-stage lung adenocarcinoma. Onco Targets Ther 2016;9:4583-91. [Crossref] [PubMed]
- Shiono S, Yanagawa N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact Cardiovasc Thorac Surg 2016;23:567-72. [Crossref] [PubMed]
- Kadota K, Sima CS, Arcila ME, et al. KRAS Mutation Is a Significant Prognostic Factor in Early-stage Lung Adenocarcinoma. Am J Surg Pathol 2016;40:1579-90. [Crossref] [PubMed]
- Enokida Y, Shimizu K, Atsumi J, et al. Prognostic potential of the MDM2 309T>G polymorphism in stage I lung adenocarcinoma. Cancer Med 2016;5:1791-801. [Crossref] [PubMed]
- Xu P, Zhao M, Liu Z, et al. Elevated nuclear CCND1 expression confers an unfavorable prognosis for early stage lung adenocarcinoma patients. Int J Clin Exp Pathol 2015;8:15887-94. [PubMed]
- Xu CH, Wang W, Wei Y, et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification in stage IB lung adenocarcinoma. Eur J Surg Oncol 2015;41:1430-6. [Crossref] [PubMed]
- Kadota K, Yeh YC, Villena-Vargas J, et al. Tumor Budding Correlates With the Protumor Immune Microenvironment and Is an Independent Prognostic Factor for Recurrence of Stage I Lung Adenocarcinoma. Chest 2015;148:711-21. [Crossref] [PubMed]
- Kadota K, Buitrago D, Lee MC, et al. Tumoral CD10 expression correlates with high-grade histology and increases risk of recurrence in patients with stage I lung adenocarcinoma. Lung Cancer 2015;89:329-36. [Crossref] [PubMed]
- Yang X, Liu Y, Lian F, et al. Lepidic and micropapillary growth pattern and expression of Napsin A can stratify patients of stage I lung adenocarcinoma into different prognostic subgroup. Int J Clin Exp Pathol 2014;7:1459-68. [PubMed]
- Noro R, Honda K, Tsuta K, et al. Distinct outcome of stage I lung adenocarcinoma with ACTN4 cell motility gene amplification. Ann Oncol 2013;24:2594-600. [Crossref] [PubMed]
- Kaseda K, Ishii G, Aokage K, et al. Identification of intravascular tumor microenvironment features predicting the recurrence of pathological stage I lung adenocarcinoma. Cancer Sci 2013;104:1262-9. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sarkaria IS, et al. Thyroid transcription factor-1 expression is an independent predictor of recurrence and correlates with the IASLC/ATS/ERS histologic classification in patients with stage I lung adenocarcinoma. Cancer 2013;119:931-8. [Crossref] [PubMed]
- Akagi I, Okayama H, Schetter AJ, et al. Combination of protein coding and noncoding gene expression as a robust prognostic classifier in stage I lung adenocarcinoma. Cancer Res 2013;73:3821-32. [Crossref] [PubMed]
- Yamauchi M, Yamaguchi R, Nakata A, et al. Epidermal growth factor receptor tyrosine kinase defines critical prognostic genes of stage I lung adenocarcinoma. PLoS One 2012;7:e43923. [Crossref] [PubMed]
- Ryu JS, Choi CM, Yang SC, et al. Prognostic effect of age on survival of patients with stage I adenocarcinoma of the lung. Tumori 2012;98:99-104. [Crossref] [PubMed]
- Kato T, Ishikawa K, Aragaki M, et al. Angiolymphatic invasion exerts a strong impact on surgical outcomes for stage I lung adenocarcinoma, but not non-adenocarcinoma. Lung Cancer 2012;77:394-400. [Crossref] [PubMed]
- Ito M, Ishii G, Nagai K, et al. Prognostic impact of cancer-associated stromal cells in patients with stage I lung adenocarcinoma. Chest 2012;142:151-8. [Crossref] [PubMed]
- Shiono S, Abiko M, Okazaki T, et al. Positron emission tomography for predicting recurrence in stage I lung adenocarcinoma: standardized uptake value corrected by mean liver standardized uptake value. Eur J Cardiothorac Surg 2011;40:1165-9. [PubMed]
- Funai K, Sugimura H, Morita T, et al. Lymphatic vessel invasion is a significant prognostic indicator in stage IA lung adenocarcinoma. Ann Surg Oncol 2011;18:2968-72. [Crossref] [PubMed]
- Seki N, Takasu T, Sawada S, et al. Prognostic significance of expression of eukaryotic initiation factor 4E and 4E binding protein 1 in patients with pathological stage I invasive lung adenocarcinoma. Lung Cancer 2010;70:329-34. [Crossref] [PubMed]
- Anagnostou VK, Syrigos KN, Bepler G, et al. Thyroid transcription factor 1 is an independent prognostic factor for patients with stage I lung adenocarcinoma. J Clin Oncol 2009;27:271-8. [Crossref] [PubMed]
- Mizuno T, Ishii G, Nagai K, et al. Identification of a low risk subgroup of stage IB lung adenocarcinoma patients. Lung Cancer 2008;62:302-8. [Crossref] [PubMed]
- Sakao Y, Tomimitsu S, Takeda Y, et al. Carcinoembryonic antigen as a predictive factor for postoperative tumor relapse in early-stage lung adenocarcinoma. Eur J Cardiothorac Surg 2004;25:520-2. [Crossref] [PubMed]
- Pelosi G, Barisella M, Pasini F, et al. CD117 immunoreactivity in stage I adenocarcinoma and squamous cell carcinoma of the lung: relevance to prognosis in a subset of adenocarcinoma patients. Mod Pathol 2004;17:711-21. [Crossref] [PubMed]
- Isobe T, Hiyama K, Yoshida Y, et al. Prognostic significance of p53 and ras gene abnormalities in lung adenocarcinoma patients with stage I disease after curative resection. Jpn J Cancer Res 1994;85:1240-6. [Crossref] [PubMed]
- Amin MB, Tamboli P, Merchant SH, et al. Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. Am J Surg Pathol 2002;26:358-64. [Crossref] [PubMed]
- Miyoshi T, Satoh Y, Okumura S, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol 2003;27:101-9. [Crossref] [PubMed]
- Kamiya K, Hayashi Y, Douguchi J, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol 2008;21:992-1001. [Crossref] [PubMed]
- Makimoto Y, Iwasaki H, Miyoshi T, et al. Micropapillary pattern: a distinct pathological marker to subclassify tumours with a significantly poor prognosis within small peripheral lung adenocarcinoma with mixed bronchioloalveolar and invasive subtypes. Histopathology 2005;46:677-84. [Crossref] [PubMed]
- De Oliveira Duarte Achcar R, Nikiforova MN, Yousem SA. Micropapillary lung adenocarcinoma: EGFR, K-ras, and BRAF mutational profile. Am J Clin Pathol 2009;131:694-700. [Crossref] [PubMed]


