Bronchoscopic assessment of bronchial anastomosis by visualizing local circulation status—index of hemoglobin (IHb) imaging
Introduction
Bronchoplasty is a common method to preserve respiratory function, and exhibits a low postoperative mortality and equal radicality to pneumonectomy in lung cancer surgery (1-3). However, anastomotic complications are occasionally experienced, potentially due to local ischemia or hypoxia as well as technical problems (1-4). It is important to observe the anastomotic conditions, although a standard assessment method has yet to be established. We performed bronchoscopy after bronchoplasty to evaluate the anastomosis and detect signs of complications at anastomosis. Ludwig and Stoelben (5), Park et al. (6) developed classification schemes to assess anastomosis using bronchoplasty, however, evaluating ischemia or hypoxia of the anastomosis by the conventional white light (WL) mode of bronchoscopy remains difficult.
The index of the hemoglobin (IHb) is a mode of endoscopic observation that visualizes levels of mucosal circulation (7-9). In the field of gastrointestinal endoscopy, the IHb image was reported to be useful in the early detection of gastric cancer, colorectal cancer and Helicobacter pylori infection by differentiating the circulation condition of mucosa (10-13). We applied this technique to detect the mucosal circulation at bronchial anastomosis, and to investigate its clinical significance for prediction of anastomosis-related morbidity.
Methods
Patients
We retrospectively reviewed the medical records of 40 patients who consecutively underwent bronchoplasty at Chiba University Hospital between January 2006 and April 2015. The patients were divided into 2 cohorts, one of which was examined as a training cohort to determine the cut-off values of the IHb to discriminate cases with and without complications, another of which was examined as a validation cohort. In the training cohort, 25 patients underwent bronchoscopy to assess the condition of the anastomosis around 2 weeks after surgery, and the relationship between IHb parameters and occurrence of anastomosis-related complications was analyzed. In the validation cohort, 15 patients underwent bronchoscopy to assess the condition of the anastomosis on POD 1, 7, and 14, and were analyzed for the IHb parameters and occurrence of anastomosis-related complications using the cut-off values obtained by the training cohort. The study protocol was approved by the Ethics Committee of Chiba University (No. 2158). The need for written informed consent was waived due to the retrospective nature of this chart review.
Technique of bronchoplasty
Standard interrupted sutures were utilized for each anastomosis using 4-0 polydioxanone suture (PDS II®, Ethicon, New Brunswick, Canada). Each suture was employed with a pitch of 2–3 mm and a bite of one cartilage depth. The first suture was placed at the deepest point from the surgeons, and in some cases knots of the first several sutures were made on the intraluminal side. As for the membranous portion, a continuous suture was also utilized. All of the cases except for lung transplantation underwent covered anastomosis using pedicle pericardial fat or intercostal muscle flap.
Bronchoscopy procedures
Flexible fiber optic bronchoscopy (BF-260, Olympus, Tokyo, Japan) and a high vision system (ELVIS LUCERA ELITE®, CV-290, Olympus, Tokyo, Japan) were used. The condition of anastomoses was assessed using both modes of WL and the IHb. Bronchoscopy was performed by thoracic surgeons under anesthesia with midazolam or short-acting benzodiazepine. All steps from the start of the pharyngeal anesthesia were completed within 30 minutes.
IHb mode
The IHb mode of bronchoscopy that is built in the high vision system can produce two-dimensional images of the mucosal blood flow by calculating the red views (Vr) and green views (Vg) (7). The IHb was calculated for each pixel of the electronic endoscopic images by logarithmic transformation of the Vr/Vg ratio using the following equation: IHb = 32[log2 (Vr/Vg)] (8), where Vr indicates the signal brightness for red (wavelength near 650 nm, showing minimal absorption by Hb) and Vg indicates the signal brightness for green (wavelength near 560 nm, showing maximal absorption by Hb) (9). The IHb mode is easily activated by pushing a button of the high vision system during broncho fiber observation.
Evaluation of the anastomosis
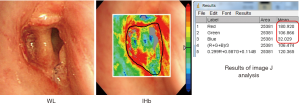
For each case, we conducted a retrospective analysis of the conventional WL images and IHb color enhanced images using the electronic endoscopic image data. The WL images were classified according to Ludwig’s (5) classification of tracheobronchial anastomosis. The analyzed IHb data were expressed by color images of red (R), green (G), and blue (B) (RGB) around the anastomosis view. The distribution area of the IHb color images were analyzed using the Image J software program (NIH, Bethesda, MD, USA) (14-16) (Figure S1).
Statistical analysis
Statistical analyses were performed using the StatMate® IV (GraphPad Software, Inc. La Jolla, CA, USA) and JMP® Pro version 12 software programs (SAS Institute Inc., Cary, NC, USA). In the training cohort, we compared several clinical factors using the Mann-Whitney U test. P values of <0.05 were considered to indicate statistical significance. We calculated receiver operating characteristic (ROC) curves for the distributions of IHb red and blue. The optimal cut-off value, with the highest Youden Index, would be the point nearest the top of the vertical axis in the ROC. In the validation cohort, we verified the accuracy of the IHb red and blue threshold values (89.2 and 109.0, respectively)—as determined in the training cohort—in the detection of anastomotic complications using the chi-squared test.
Results
Analysis of the training cohort
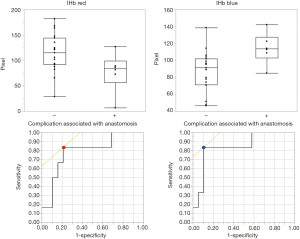
Bronchoplasty was performed on 25 patients, consisting of 19 men and 6 women. Fifteen cases were right-sided bronchoplasty, and 10 cases were left-sided (Table 1). The anastomosis-related complications were three cases of air leakage, two pneumonia, and one empyema with bronchopleural fistula (BFP) (Table S1). The IHb findings of the cases with anastomosis-related complications demonstrated narrower red distributions and wider blue distributions than those without complications. Some cases showed a discrepancy between the WL classification and IHb RGB distribution (Figure 1). We compared several clinical factors, WL classification (5), and IHb RGB distribution in Table 2 between patients without complications (n=19) and with complications (n=6). There were no significant differences in age, sex, basic disease, induction therapy, or WL classification. However, there were significant differences in the IHb red and blue distributions (P=0.03, P=0.01, respectively) (Table 2). We analyzed the ROC curve of the IHb red and blue distributions (Figure 2). The IHb red threshold was 89.2 for whether a complication had occurred (sensitivity 83.3%, specificity 62.2%). The IHb blue threshold was 109.0 for whether a complication had occurred (sensitivity 83.3%, specificity 72.8%).

Full table
Full table
Full table
Analysis of the validation cohort
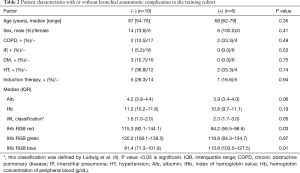
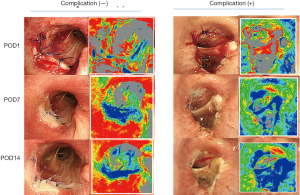
The operative procedures consisted of 15 bronchoplasty including 2 lung transplant recipients and 2 living lung donors. There were nine cases of right-sided bronchoplasty, seven left-sided, and one case of carinaplasty (Table 3). Six patients had complications (Table S2). The anastomosis-related complications were three cases of granulation, two pneumonia with stenosis, and one anastomotic leakage. The mucosal ischemic changes were much clearer after the IHb color enhancement. Two representative cases are shown in Figure 3. Case 2 developed pneumonia with stenosis due to mucosal ischemic changes in the peripheral side from the anastomosis. On POD 1, neither the WL nor IHb images showed mucosal ischemia around the anastomosis. On POD 14, both WL and IHb images showed the presence of mucosal ischemic changes. Interestingly, On POD 7, the WL image failed to show the mucosal ischemia, but the IHb image revealed the spread of IHb blue distribution around the anastomosis compared to the image on POD 1. The IHb image was therefore able to detect mucosal ischemic changes earlier than the WL image.
Full table

Full table
The accuracy of the IHb red and blue threshold values (89.2 and 109.0, respectively) as determined in the training cohort to detect anastomotic complications was verified (Table 4). The IHb red distribution was able to detect the complication in only one case on POD 1 (16.7%), whereas the IHb blue was able to detect 2 cases with complications on POD 1 (33.3%). On POD 7, the IHb blue distribution was able to detect 3 cases with complications with a specificity of 90.0% and sensitivity of 60.0%. The IHb red and blue distributions on POD 14 had sensitivities of 60.0% and specificities of 75.0%. These results indicate that the IHb blue distribution on POD 7 is potentially useful for prediction of complications associated with the anastomosis at the early healing phase.

Full table
Discussion
This retrospective study assessed the ability of IHb to detect anastomotic complications caused by ischemia in patients treated with bronchoplasty following radical surgery for lung cancer. The complications associated with anastomosis after bronchoplasty have been reported to occur in 4.0–22.4% of patients (1-4). The circulation and oxygenation at bronchial anastomosis is strongly related to occurrence of the complications. Concerning sleeve lobectomy, it has been accepted that the blood flow at anastomosis is supplied by the peripheral lung (17), while regarding lung transplantation, the blood supply at the bronchial anastomosis is dependent on the central bronchial artery and distal pulmonary artery (18,19).
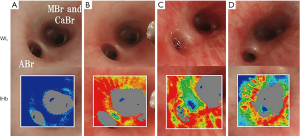
In a preliminary experiment, we confirmed the IHb color change in relation to mucosal microcirculation using specific pathogen-free swine (SPF) 1 mL of isosorbide mononitrate (1 mg isosorbide mononitrate in 1 mL Nitorol®; Eisai, Tokyo, Japan) was sprayed on the bronchial mucosa through a bronchoscope (BF-260, Olympus, Tokyo, Japan), and observed periodically (20). The WL and IHb observations were subsequently compared. After spraying 1 mL of isosorbide mononitrate onto the right bronchial mucosa in SPF swine, the mucosa immediately changed to a reddish color in the WL images, and the red area spread in the IHb images (Figure 4). Forty-five minutes later, the mucosal redness faded. We were able to confirm that the changes that were visualized in the bronchial mucosal circulation were in line with those reported in the gastrointestinal mucosa (7-9).
In this study, the IHb was used as a surrogate of local circulation of the bronchial mucosa, and a preliminary animal study clearly demonstrated the theoretical function of the IHb. Then, the relationship between the IHb finding and anastomosis-related complications was investigated. Among the complications, prolonged air leakage and pneumonia were noted, which were not directly associated with the anastomotic condition. However, such pulmonary complications have an inherent indirect association with anastomosis, since ischemia or necrosis of the bronchial mucosa critically affects not only anastomotic healing but also peripheral condition. As a result, the distribution of IHb blue on POD 7 exhibited the closest association with the occurrence of the complications, and an IHb assessment on POD 7 could predict serious complications, such as BPF or stenosis.
Several modalities for assessing bronchial anastomosis have been reported in cases with lung transplantation (21). Bronchoscopic narrow-band imaging (NBI) mode and auto fluorescence imaging (AFI) have been used to assess airway vascularity after lung transplantation (22,23). The NBI mode is an endoscopic technique that uses optical filters to narrow the bandwidth. However, the previous study did not report the anastomosis conditions and complications (22). AFI bronchoscopy visualized the intensity and spectral contrast of the auto fluorescence of the healthy bronchial mucosa and neoplastic or early cancerous lesions. Investigators produced airway ischemic damage in experimental swine models and compared the histological results and AFI findings (23). The bronchoscopic AFI mode can potentially detect the airway ischemic damage, however, the clinical patient examinations were not reported. Laser Doppler Flowmetry (LDF) was also reported to be useful for evaluating bronchial anastomosis of swine (20,24). LDF is a technique that involves a narrow beam of monochromatic light that hits moving blood cells and allows the blood flow to be detected. Clinical human cases, however, were not published.
To the best of our knowledge, we are the first to report the use of IHb images in assessing the anastomosis following bronchoplasty. The IHb mode is very simple to use and has the potential to assess the mucosal blood flow.
The most significant contribution of the study to the field of general thoracic surgery is to offer an objective and reproducible method to assess healing of tracheobronchial anastomosis. Tracheobronchial surgery comprises various modifications such as complete continuous sutures (25), the combination of interrupted and continuous sutures, telescopic anastomosis sutures (26), and figure-of-eight suture (27). The covering of anastomosis remains controversial in terms of its effectiveness or the types of utilized tissues, such as the pedicled pericardial fat pad (28), thymic tissue (29) and intercostal muscle flaps (30). IHb images can therefore be used to evaluate and compare the above procedures. However, The IHb assessment must cover bronchial stump healing.
There are several limitations associated with our study. First, we defined the anastomosis-related complications in the training cohort as air leakage, pneumonia, and empyema with fistula. We were, however, unable to distinctly prove these complications or anastomotic mucosal conditions. Second, it was difficult to assess bronchial anastomosis just after the surgery due to mucosal edema or blood clots. Third, the number of patients in this study cohort was small. Thus, a larger cohort study is necessary to confirm our findings.
In conclusion, the present study showed that IHb images were useful for assessing the condition of the anastomosis after bronchoplasty. IHb images are simple to obtain and may be helpful in the diagnosis of mucosal ischemic damage of bronchial anastomosis after bronchoplasty.
Acknowledgements
None.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Okada M, Yamagishi H, Satake S, et al. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. J Thorac Cardiovasc Surg 2000;119:814-9. [Crossref] [PubMed]
- Merritt RE, Mathisen DJ, Wain JC, et al. Long-term results of sleeve lobectomy in the management of non-small cell lung carcinoma and low-grade neoplasms. Ann Thorac Surg 2009;88:1574-81; discussion 1581-2. [Crossref] [PubMed]
- Tagawa T, Iwata T, Nakajima T, et al. Evolution of a Lung-Sparing Strategy with Sleeve Lobectomy and Induction Therapy for Non-small Cell Lung Cancer: 20-Year Experience at a Single Institution. World J Surg 2016;40:906-12. [Crossref] [PubMed]
- Ludwig C, Stoelben E, Olschewski M, et al. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73. [Crossref] [PubMed]
- Ludwig C, Stoelben E. A new classification of bronchial anastomosis after sleeve lobectomy. J Thorac Cardiovasc Surg 2012;144:808-12. [Crossref] [PubMed]
- Park SY, Lee HS, Jang HJ, et al. Wedge bronchoplastic lobectomy for non-small cell lung cancer as an alternative to sleeve lobectomy. J Thorac Cardiovasc Surg 2012;143:825-31.e3. [Crossref] [PubMed]
- Nakamura K. Development of real-time endoscopic image processing technology: adaptive index of hemoglobin color enhancement processing. Digestive Endoscopy 2002;14:S40-7. [Crossref]
- Tsuji S, Sato N, Kawano S, et al. Functional imaging for the analysis of the mucosal blood hemoglobin distribution using electronic endoscopy. Gastrointest Endosc 1988;34:332-6. [Crossref] [PubMed]
- Kim GH, Kim KB, Lim EK, et al. Analysis of endoscopic electronic image of intramucosal gastric carcinoma using a software program for calculating hemoglobin index. J Korean Med Sci 2006;21:1041-7. [Crossref] [PubMed]
- Yao K, Yao T, Matsui T, et al. Hemoglobin content in intramucosal gastric carcinoma as a marker of histologic differentiation: a clinical application of quantitative electronic endoscopy. Gastrointest Endosc 2000;52:241-5. [Crossref] [PubMed]
- Kobayashi K, Igarashi M, Sada M, et al. Clinical significance of adaptive index of hemoglobin color enhancement for endoscopic diagnosis of superficial type colorectal tumors. Dig Endosc 2002;14:S51-3. [Crossref]
- Kato M, Terao S, Adachi K, et al. Changes in endoscopic findings of gastritis after cure of H. pylori infection: multicenter prospective trial. Dig Endosc 2013;25:264-73. [Crossref] [PubMed]
- Uchiyama K, Ida K, Okuda J, et al. Correlations of hemoglobin index (IHb) of gastric mucosa with Helicobacter pylori (H. pylori) infection and inflammation of gastric mucosa. Scand J Gastroenterol 2004;39:1054-60. [Crossref] [PubMed]
- Rasband WS, Image J. U. S. National Institutes of Health, Bethesda, Maryland, USA. Available online: http://imagej.nih.gov/ij/
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671-5. [Crossref] [PubMed]
- Abramoff MD, Magelheas PJ, Ram SJ. Image Processing with Image J. Biophotonics International 2004;11:36-47.
- Herold U, Jakob H, Kamler M, et al. Interruption of bronchial circulation leads to a severe decrease in peribronchial oxygen tension in standard lung transplantation technique. Eur J Cardiothorac Surg 1998;13:176-83. [Crossref] [PubMed]
- Nicolls MR, Zamora MR. Bronchial blood supply after lung transplantation without bronchial artery revascularization. Curr Opin Organ Transplant 2010;15:563-7. [Crossref] [PubMed]
- Inui K, Wada H, Yokomise H, et al. Evaluation of a bronchial anastomosis by laser Doppler velocimetry. J Thorac Cardiovasc Surg 1990;99:614-9. [PubMed]
- Nakakuki S. Bronchial tree, lobular division and blood vessels of the pig lung. J Vet Med Sci 1994;56:685-9. [Crossref] [PubMed]
- Yserbyt J, Dooms C, Vos R, et al. Anastomotic airway complications after lung transplantation: risk factors, treatment modalities and outcome-a single-centre experience. Eur J Cardiothorac Surg 2016;49:e1-8. [Crossref] [PubMed]
- Irani S, Thuer I, Seifert B, et al. Endoscopic narrow-band imaging-quantitative assessment of airway vascularity after lung transplantation. J Biomed Opt 2009;14:014010. [Crossref] [PubMed]
- Iga N, Oto T, Okada M, et al. Detection of airway ischaemic damage after lung transplantation by using autofluorescence imaging bronchoscopy. Eur J Cardiothorac Surg 2014;45:509-13. [Crossref] [PubMed]
- Korpela A, Aarnio P, Harjula A. Evaluation of the bronchial mucosal blood flow by layser doppler flowmeter. Int J Angiol 1995;4:110-2. [Crossref]
- Yang R, Shao F, Cao H, et al. Bronchial anastomosis using complete continuous suture in video-assisted thoracic surgery sleeve lobectomy. J Thorac Dis 2013;5 Suppl 3:S321-2. [PubMed]
- Hollaus PH, Janakiev D, Pridun NS. Telescope anastomosis in bronchial sleeve resections with high-caliber mismatch. Ann Thorac Surg 2001;72:357-61. [Crossref] [PubMed]
- Date H, Trulock EP, Arcidi JM, et al. Improved airway healing after lung transplantation. An analysis of 348 bronchial anastomoses. J Thorac Cardiovasc Surg 1995;110:1424-32; discussion 1432-3. [Crossref] [PubMed]
- Asamura H, Kondo H, Tsuchiya R. Management of the bronchial stump in pulmonary reections: a review of 533 consecutive recent bronchial closures. Eur J Cardiothorac Surg 2000;17:106-10. [Crossref] [PubMed]
- Infante MV, Alloisio M, Balzarini L, et al. Protection of right pneumonectomy bronchial sutures with a pedicled thymus flap. Ann Thorac Surg 2004;77:351-3. [Crossref] [PubMed]
- Bylicki O, Vandemoortele T, Orsini B, et al. Incidence and management of anastomotic complications after bronchial resection: a retrospective study. Ann Thorac Surg 2014;98:1961-7. [Crossref] [PubMed]


