Induction chemoradiotherapy versus induction chemotherapy for potentially resectable stage IIIA (N2) non-small cell lung cancer: a systematic review and meta-analysis
Introduction
Nowadays, lung cancer is the most common cancer diagnosed worldwide and the leading cause of cancer-related deaths (1). Non-small cell lung cancer (NSCLC) accounts for 85% to 90% of lung cancer cases (2). At diagnosis, around 30% of NSCLC patients are already at stage IIIA (N2). Currently, definitive concurrent chemoradiation remains as the main treatment strategy for stage IIIA NSCLC. However, for potentially resectable stage IIIA (N2) NSCLC, induction therapy (also known as neoadjuvant therapy) followed by surgery is recommended as an alternative. Induction therapy includes chemotherapy alone, sequential chemoradiation, concurrent chemoradiation and concurrent chemoradiation after chemotherapy. For stage IIIA (N2) NSCLC, several studies have shown that induction chemotherapy followed by surgery improved survival, compared with surgery alone (3-5). Meanwhile, induction by concurrent chemoradiotherapy followed by surgery resulted in 5-year survival rates of 30% to 40%, appearing superior to surgery alone (6-8). However, a consensus has not been reached on which induction therapy should be administrated to stage IIIA (N2) patients—50% of the National Comprehensive Cancer Network (NCCN) member institutions choose induction chemoradiotherapy, while another 50% choose induction chemotherapy (9).
A recently published phase III randomized clinical trial evaluated the survival benefit of induction chemoradiotherapy versus induction chemotherapy followed by surgery for stage IIIA (N2) patients, showing that radiotherapy did not add any benefit. The authors suggest that induction chemotherapy followed by surgery is an adequate treatment option for stage IIIA (N2) NSCLC (10). To incorporate currently available data to better evaluate the efficacy and toxicity of addition of radiotherapy to induction chemotherapy followed by surgery for stage IIIA (N2) NSCLC patients, we carried out a systematic review and meta-analysis.
Methods
Eligibility criteria
The following criteria for eligibility were set before searching for studies: (I) patients had pathologically proven, locally advanced T1-3N2M0, stage IIIA (N2) NSCLC based on the TNM classification; (II) the trials were randomized clinical trials of induction chemoradiotherapy versus induction chemotherapy followed by surgery; (III) studies reported any of the following information: tumor responses, pathological complete response (pCR) of mediastinal lymph nodes, adverse reactions, overall survival (OS) and progression-free survival (PFS); (IV) the full-text articles were published in English. The exclusion criteria were: (I) letters, meeting abstracts, reviews and case reports; (II) duplicate reports of the same patient cohorts; (III) studies without useful data or endpoints.
Search strategy
A comprehensive literature search was conducted to identify all relevant studies. PubMed, Embase, Web of Science and Cochrane Library were systematically searched for studies published from the inception of each database to September 10, 2017. The following key words were used in the search strategy: “non-small cell lung cancer” AND “IIIA (N2)” AND “induction therapy”. In addition, the search was extended to identify any other potential studies by review of references of articles included in the final selection.
Data extraction
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement to report the materials and methods (11). Two investigators independently searched for eligible studies and extracted the data. Disagreements between investigators on data extraction were resolved through discussion with a third investigator. For the meta-analysis, the following data were extracted: objective response rate (ORR), pCR rate of mediastinal lymph nodes, incidence of grade 3–4 adverse events (nausea and vomiting, infections, leukopenia and anemia), the hazard ratio (HR) with a 95% confidence interval (CI) for OS and PFS. Meanwhile, the following information of the eligible studies was recorded: author, year, study period, groups, number of patients and treatment strategies.
Risk of bias assessment
Assessment of risk of bias was performed according to the Cochrane Collaboration’s tool (12). Two investigators evaluated risk of bias of all eligible studies by random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias. Each domain of bias was classified as “high risk”, “unclear risk” or “low risk”. The risk of bias of a trial was rated “high” when any of the key domains was at high risk of bias; conversely, it was rated “low” when all the key domains were at low risk of bias; otherwise, it was rated “unclear”.
Statistical analyses
Statistical analyses were performed using Review Manager v5.3 (RevMan, The Cochrane Collaboration). Primary outcome measures were odds ratios (ORs) for ORR, risk ratios (RRs) for grade 3–4 adverse events, HRs for OS and PFS. 95% CI was used for each endpoint. P value of <0.05 was considered significant. Statistical heterogeneity was evaluated using the Cochrane χ2 test and the I2 statistic. I2<50% was considered as low heterogeneity, whereas I2≥50% was considered as potentially significant heterogeneity. The meta-analysis was performed either via a random effects model or a fixed effects model according to the degree of heterogeneity. A random effects model was used in case of potentially significant heterogeneity (P<0.10, I2 ≥50%); otherwise, a fixed effects model was used.
Results
Study selection
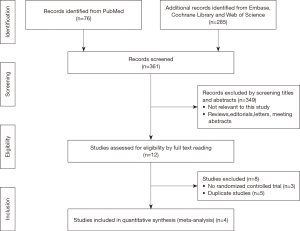
As summarized in Figure 1, 361 records of relevant studies were obtained from PubMed (n=76) and other databases (n=285). Twelve studies passed the screening of title and abstract. After full text screening, eight studies were excluded due to nonrandomized controlled trials or duplicate data. Finally, four randomized controlled trials, containing 461 patients, were included in this meta-analysis (10,13-15).
Characteristics of the included studies
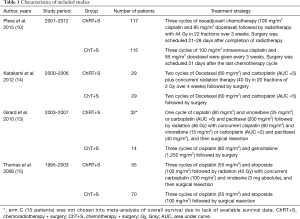
These studies were conducted in Japan, Germany, Switzerland, Serbia and France and published between 2008 and 2015. All the IIIA (N2) NSCLC patients were divided into intervention groups (induction chemoradiotherapy followed by surgery, ChRT+S) and control groups (induction chemotherapy followed by surgery, ChT+S). The characteristics and the treatment schedules were listed in Table 1.
Full table
Risk of bias in included studies
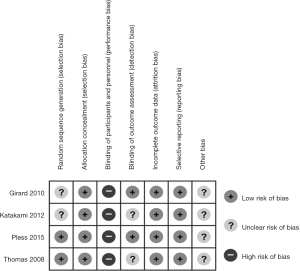
The quality of the studies was evaluated by risk of bias assessment. Two studies described the methods of generating random assignment sequence in detail (10,15), while the other two did not (13,14). Allocation concealment was performed adequately in all four studies. Blinding of participants and personnel was difficult to achieve due to the nature of these studies, of which the risk of bias was high. Blinding of outcome assessment was described in two studies (10,13), but not in other two studies (14,15). The outcome data of all four studies were complete and objective, and the risk of attrition bias, as well as selective reporting bias, was low. Other potential biases such as specific study design and fraudulent action were not clear in all the studies. Overall, the methodological quality of these studies was good. The details of risk of bias assessment were summarized in Figure 2.
Tumor responses
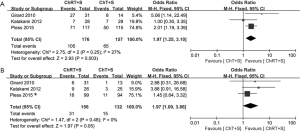
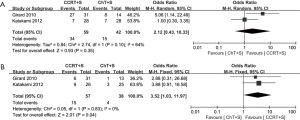
WHO criteria were used to assess tumor responses (16). ORR after induction treatment was reported in three studies (10,13,14). A fixed effects model was adopted, as no significant heterogeneity was observed (χ2=2.75, P=0.25, I2=27%). The pooled ORR in the intervention group was significantly higher than that in the control group (OR =1.97, 95% CI: 1.25–3.10, P<0.05) (Figure 3A).
pCR of mediastinal lymph nodes
Three studies provided data for pCR of mediastinal lymph nodes (10,13,14). A fixed effect model was used, as no significant heterogeneity was observed (χ2=1.47, P=0.48, I2=0%). The meta-analysis revealed an improved pCR rate of mediastinal lymph nodes in the intervention group (OR =1.97, 95% CI: 1.00–3.86, P=0.05) (Figure 3B).
Adverse reactions
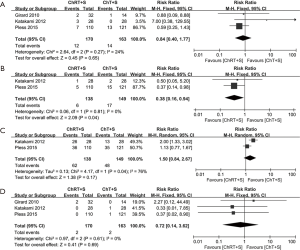
Nausea and vomiting, infections, leukopenia and anemia were included as adverse reactions in this meta-analysis. We focused on grade 3–4 adverse reactions, as they often require intervention in clinical practice. Grade 3–4 nausea and vomiting data from three studies (10,13,14) were incorporated into this meta-analysis. No significant heterogeneity was observed (χ2=2.64, P=0.27, I2=24%). There was no significant difference between the two groups (RR =0.84, 95% CI: 0.40–1.77, P=0.65) (Figure 4A). Grade 3–4 infections data from two studies (10,14) were incorporated into this meta-analysis. No significant heterogeneity was observed (χ2=0.06, P=0.81, I2=0%). It appears that the incidence of infections in the intervention group was lower than that in the control group (RR =0.38, 95% CI: 0.16–0.94, P=0.04) (Figure 4B); however, this was very likely an artifact (see in discussion). Grade 3–4 leukopenia data from two studies (10,14) were incorporated into this meta-analysis. A random effects model was adopted due to statistically significant heterogeneity (χ2=4.17, P=0.04, I2=76%). There was no significant difference between the two groups (RR =1.50, 95% CI: 0.84–2.67, P=0.17) (Figure 4C). Grade 3–4 anemia data from three studies (10,13,14) were incorporated into this meta-analysis. No significant heterogeneity was observed (χ2=0.97, P=0.61, I2 =0%). There was no significant difference between the two groups (RR =0.72, 95% CI: 0.14–3.62, P=0.69) (Figure 4D).
Survival
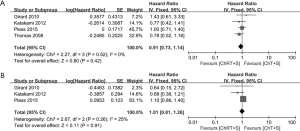
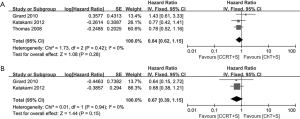
Survival was analyzed based on OS and PFS. OS was reported in all four studies with a total of 446 patients (10,13-15), no significant heterogeneity was observed (χ2=2.27, P=0.52, I2=0%), and there was no significant difference concerning OS between the two groups (HR =0.91, 95% CI: 0.73–1.14, P=0.42) (Figure 5A). PFS from three studies (10,13,14) were incorporated into the pooled analysis, no significant heterogeneity was observed (χ2=2.67, P=0.26, I2 =25%), and also there was no significant difference concerning PFS between the two groups (HR =1.01, 95% CI: 0.81–1.26, P=0.91) (Figure 5B). Of note, a fixed effects model was adopted in all the meta-analysis for survival data.
Discussion
Over the past decades, several studies have demonstrated that preoperative induction treatment plus surgery, compared with surgery alone, significantly improved the survival of stage IIIA (N2) NSCLC patients (3-5,17). However, the optimal induction treatment for potentially resectable stage IIIA (N2) NSCLC remains unclear. Particularly, whether induction chemoradiotherapy is superior to induction chemotherapy has not been clearly demonstrated.
In this work, we attempted to resolve this issue by a systematic review and meta-analysis, which may be helpful for clinicians to determine the optimal induction treatment. We compared induction chemoradiotherapy with induction chemotherapy from four aspects: tumor responses, pCR of mediastinal lymph nodes, adverse reactions and survival data. Our meta-analysis suggests that induction chemoradiotherapy indeed improved ORR and pCR rate of mediastinal lymph nodes. Unexpectedly, our meta-analysis indicates that induction chemoradiotherapy decreased the incidence of grade 3–4 infection. However, after having carefully inspected the two studies, we conclude that this result was not credible. Because in the WJTOG9903 study (14), wherein concurrent chemoradiotherapy was performed in the intervention group, there was no significant difference in the incidence of grade 3–4 infection between the two groups; while in the SAKK trial (10), radiotherapy alone actually did not increase the risk of grade 3–4 infection, thereby mitigating the incidence of infection in the intervention group. Importantly, our meta-analysis indicates that induction chemoradiotherapy did not result in an improved OS or PFS, compared with induction chemotherapy. A similar conclusion has been reached by two previous meta-analyses (18,19), which included different trials thus different patient cohorts, and two retrospective studies (9,20).
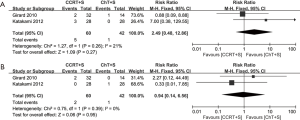
Of note, in the most weighted SAKK trial (10), before surgery, sequential chemoradiotherapy was conducted, whereas concurrent chemoradiotherapy was conducted in the other three trials (10,13-15). We also performed the meta-analyses without the SAKK trial to evaluate concurrent chemoradiotherapy versus chemotherapy, while the conclusions on pCR rate of mediastinal lymph nodes, OS and PFS remain unchanged, concurrent chemoradiotherapy showed a trend to improve ORR, but the difference did not reach statistical significance, due to only two trials with small cohorts and inconsistent data on this issue were included (Figure S1-S3). Meta-analysis for the incidence of grade 3–4 infection or leukopenia was not conducted, due to only one trial could be included.
Compared with two previous meta-analyses (18,19), our meta-analysis was completely based on high-quality randomized clinical trials and included the latest study not available for previous meta-analysis. Moreover, unlike previous meta-analyses that only included survival analysis, our meta-analysis also included tumor responses, pCR of mediastinal lymph nodes and adverse reactions.
Meanwhile, our meta-analysis also has several drawbacks. First, blinding method was not adopted in all four randomized clinical trials due to practical difficulties, therefore performance bias was inevitable. Second, the differences in the regimens/protocols of radiotherapy and/or chemotherapy in all included studies might lead to certain degree of clinical heterogeneity, which might affect the outcome of the analysis. Third, only a small number of studies were included in the meta-analysis, and some studies had relatively small sample sizes, therefore weakened the strength of the meta-analysis.
More importantly, the conclusions of our meta-analysis are dictated by the quality of the included data. In the SAKK trial by Pless et al. (10), a high percentage of the patients (~16% of each arm) did not receive the allocated treatment. Also, the high weight of this trial has a great impact on the results of our meta-analysis. In the WJTOG9903 study by Katakami et al. (14), the insufficient radiation dose and the short interval between induction therapy and surgery resulted in a low response rate to induction chemoradiotherapy (~25% vs. ~65% in average in other concurrent chemoradiotherapy studies) (6-8). In the French IFTC-0101 trial by Girard et al. (13), median survival was not reached in arm C (one of the two induction chemoradiotherapy groups) due to insufficient follow-up time, which could have affected the OS analysis. In the GLCCG trial by Thomas et al. (15), both stage IIIA and IIIB NSCLC patients were included, the endpoints were not reported separately, precluding this trial from most of our analyses, despite it contained the largest cohorts.
The reason why addition of radiotherapy did not result in an improved OS or PFS may lie in the setting that only operated patients were taken into account for the analysis in both the original trials and the meta-analysis, the initial benefit from radiotherapy was likely mitigated by the following surgery. However, in reality, not all the potentially resectable stage IIIA (N2) NSCLCs turn out to be operable after the induction therapy; also, not all the surgery can achieve a complete resection of the tumors. Addition of radiotherapy may still be beneficial for such patients. Thus, before treatment, it is essential to carefully evaluate the disease stage and to discuss whether surgery is appropriate by a multidisciplinary team (21,22). If the confidence for a complete resection is high, we do not recommend including radiotherapy in the induction therapy; if the situation is opposite, radiotherapy should also be considered. More studies are required to draw a better conclusion.
Conclusions
In conclusion, induction chemoradiotherapy may have limited value concerning tumor response and pCR of mediastinal lymph nodes. Compared with induction chemotherapy, induction chemoradiotherapy does not exacerbate the toxicity. Current evidence does not support that addition of radiotherapy to induction chemotherapy followed by surgery can bring a significantly prolonged OS or PFS to operable stage IIIA (N2) NSCLC patients.
Acknowledgements
Funding: This work was supported by the “Six Talent Peaks” Project by Jiangsu Department of Human Resources and Social Security (No. 2013-WSN-082); the Project of Science and Technology by Jiangsu Provincial Commission of National Health and Family Planning (No. H201426); the Project of Xuzhou Science and Technology Bureau (No. KC15SH002); the “Six One” Project by Jiangsu Provincial Commission of National Health and Family Planning (No. LGY2016041); Jiangsu Provincial Medical Innovation Team (No. CXTDA2017034); and the Special Fund for Scientific Research in the Public Interest (No. 201402011).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2010;8:740-801. [Crossref] [PubMed]
- Rosell R, Gomez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 1994;330:153-8. [Crossref] [PubMed]
- Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. [Crossref] [PubMed]
- Choi NC, Carey RW, Daly W, et al. Potential impact on survival of improved tumor downstaging and resection rate by preoperative twice-daily radiation and concurrent chemotherapy in stage IIIA non-small-cell lung cancer. J Clin Oncol 1997;15:712-22. [Crossref] [PubMed]
- Eberhardt W, Wilke H, Stamatis G, et al. Preoperative chemotherapy followed by concurrent chemoradiation therapy based on hyperfractionated accelerated radiotherapy and definitive surgery in locally advanced non-small-cell lung cancer: mature results of a phase II trial. J Clin Oncol 1998;16:622-34. [Crossref] [PubMed]
- Thomas M, Rube C, Semik M, et al. Impact of preoperative bimodality induction including twice-daily radiation on tumor regression and survival in stage III non-small-cell lung cancer. J Clin Oncol 1999;17:1185. [Crossref] [PubMed]
- Higgins K, Chino JP, Marks LB, et al. Preoperative chemotherapy versus preoperative chemoradiotherapy for stage III (N2) non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2009;75:1462-7. [Crossref] [PubMed]
- Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015;386:1049-56. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Girard N, Mornex F, Douillard JY, et al. Is neoadjuvant chemoradiotherapy a feasible strategy for stage IIIA-N2 non-small cell lung cancer? Mature results of the randomized IFCT-0101 phase II trial. Lung Cancer 2010;69:86-93. [Crossref] [PubMed]
- Katakami N, Tada H, Mitsudomi T, et al. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer 2012;118:6126-35. [Crossref] [PubMed]
- Thomas M, Rube C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol 2008;9:636-48. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Nagai K, Tsuchiya R, Mori T, et al. A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209). J Thorac Cardiovasc Surg 2003;125:254-60. [Crossref] [PubMed]
- Shah AA, Berry MF, Tzao C, et al. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg 2012;93:1807-12. [Crossref] [PubMed]
- Xu YP, Li B, Xu XL, et al. Is There a Survival Benefit in Patients With Stage IIIA (N2) Non-small Cell Lung Cancer Receiving Neoadjuvant Chemotherapy and/or Radiotherapy Prior to Surgical Resection: A Systematic Review and Meta-analysis. Medicine (Baltimore) 2015;94:e879. [Crossref] [PubMed]
- Pezzetta E, Stupp R, Zouhair A, et al. Comparison of neoadjuvant cisplatin-based chemotherapy versus radiochemotherapy followed by resection for stage III (N2) NSCLC. Eur J Cardiothorac Surg 2005;27:1092-8. [Crossref] [PubMed]
- Farjah F, Flum DR, Varghese TK Jr, et al. Surgeon specialty and long-term survival after pulmonary resection for lung cancer. Ann Thorac Surg 2009;87:995-1004; discussion 1005-6. [Crossref] [PubMed]
- Martins RG, D’Amico TA, Loo BW Jr, et al. The management of patients with stage IIIA non-small cell lung cancer with N2 mediastinal node involvement. J Natl Compr Canc Netw 2012;10:599-613. [Crossref] [PubMed]