Minimally invasive surgery improves outcome of left ventricular assist device surgery in cardiogenic shock
Introduction
Heart failure (HF) is a constantly growing global health problem with high mortality rates (1). As cardiac transplantation is not available for all patients, ventricular assist device (VAD) surgery has become a standard therapy for HF patients (2-4). However, despite important advances in VAD therapy, perioperative mortality strongly correlates with preoperative patient status (5,6). Thus, patients in refractory cardiogenic shock are the most vulnerable group of all VAD patients (7). Therefore, an important consensus in management of congestive heart failure (CHF) is to perform VAD implantation in a compensated status, for increasing patient safety and decreasing perioperative risks (8). Nevertheless, patients refractory to medical treatment in cardiogenic shock will persist, and their management represents one of the biggest challenges in CHF therapy. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles of advanced HF were defined in the setting of a multi-institutional registry of VAD to clarify the clinical characterization of HF patients with a failed response to conventional treatment. The aims were standardization of communication between colleagues, risk management and selection of target populations for advanced therapies. In addition, INTERMACS profiles are related to patients’ prognosis following VAD implantation (9). INTERMACS level I patients, the so called “crash and burn patients”, are VAD candidates with the poorest outcome based on a combination of decompensated cardiogenic shock and low cardiac output with multi-organ failure showing tremendous perioperative risks with 1-year survival rates known to be as lower than 30% following VAD implantation (7,10). Thus, it has been demonstrated that temporary mechanical circulatory support for INTERMACS 1 patients is useful as it is less aggressive and allows gaining time for bridge to decision, heart transplantation or VAD implantation (11). However, when the decision for VAD implantation is made, these patients are still at very high operative risk. Thus avoidance of full sternotomy and novel less invasive surgical techniques might be beneficial for these patients.
Nevertheless, so far still the most commonly surgical technique used for left ventricular assist device (LVAD) implantation is the conventional technique, which involves a full sternotomy. In the past, this approach was required due to relatively large pump sizes and limited surgical experience with minimally invasive LVAD implantation procedures (12,13). However, full sternotomy is associated with high rates of adverse effects, such as major bleeding, infection, postoperative respiratory failure and long intensive care unit (ICU) and overall hospital stays (12,13). In LVAD surgery this is especially critical, since the therapy itself involves alterations of hemostasis like the acquired von Willebrand syndrome, which further increases the perioperative bleeding risk (14,15). Additionally, the full opening of the pericardium that goes along with a full sternotomy abrogates the natural confinements of the right ventricle, increasing the risk of right heart failure (RHF) once the LVAD pump is started (16). Existing literature suggests that the incidences of bleeding, requiring surgery and extended inotropic support, range between 30–40% in destination LVAD surgery (2,3). These persistent complications have reduced the clinical acceptance of LVAD therapy (17,18). On the other hand, the constant development of VADs has led to improved technologies and remarkably reduced sizes (4). This has enabled less invasive techniques for implantation, explantation and exchange of VADs (16,19). This new era of minimally invasive LVAD surgery is expected to improve outcomes by reducing critical operative complication rates like bleeding or right ventricular (RV) failure (8).
This study presents the first long-term results of INTERMACS 1 patients ineligible for heart transplant due to organ shortage, who were implanted with a HVAD continuous-flow LVAD using minimally invasive techniques for destination therapy.
Methods
Study design and patient population
This was an observational retrospective or urgency study comparing results from a cohort of the first 14 consecutive patients who underwent implantation of an HVAD continuous-flow LVAD by less invasive techniques (group A), with results from 18 historical cohort patients who underwent HVAD implantation by conventional sternotomy procedures (group B) between January 2012 to December 2014. All patients were monitored for 1 year after implantation. Patients were considered for treatment if they had advanced HF refractory to medical therapy, but were ineligible for heart transplantation due to their age. All patients were operated by the same surgical team that also made the decision of which technique was used for implantation. Patients requiring concomitant cardiac surgery (such as valve repair or bypass surgery) were excluded from the study.
Data collection
Baseline data were collected from all enrolled patients. Assessments included characteristics such as age, gender, body surface area, body mass index, HF etiology, New York Heart Association class, INTERMACS profile and history of stroke. Other baseline data included left ventricular (LV) ejection fraction, cardiac index, central venous pressure, pulmonary capillary wedge pressure, pulmonary vascular resistance, pulmonary arterial pressures and laboratory values (creatinine, C-reactive protein).
Follow-up after device implantation
Postoperative medical care (including inotropic, antiarrhythmic, anticoagulant, and HF therapy) was managed according to standard protocols.
Surgical techniques
The minimally invasive technique was performed as previously reported (16). First, the position of the LV apex was identified through transthoracic echocardiography and marked on the patient’s skin in order to perform a minimized incision for thoracotomy. Then an upper J-shaped hemisternotomy was performed followed by thoracotomy including opening the pericardium anterior to the LV apex. The sewing ring was fixed 1 cm anterior to the true apex, and after making sure that no bleeding was present at the side of the apical sewing ring, heparin was administered, as per standard protocol. In order to establish cardiopulmonary bypass, cannulas were placed into ascending aorta (arterial) and femoral vein (venous). Following LVAD insertion, the driveline was placed through the sheath of the rectus muscle in umbilical direction and then subcutaneously externalized towards to the right or left upper quadrant. Afterwards, the outflow graft was tunnelled intrapericardially towards the upper hemisternotomy and anastomosed end-to-side to the distal ascending aorta. The conventional procedure consisted of a pump and outflow graft insertion via a median sternotomy. In both cases, surgeries were performed safely using cardiopulmonary bypass.
Outcomes
The primary endpoint was survival at 1 year without re-operation to replace the originally implanted assist device. Secondary endpoints included survival at 90 days and a composite of rate of RHF, respiratory failure and significant bleeding during the 1 year of follow-up period. Other adverse events (AEs) including infection, renal failure, pump thrombosis, hepatic dysfunction and stroke were also recorded. All complications and AEs were defined according to INTERMACS registry definitions (9).
Statistical analysis
Differences between groups for independent, normally distributed and continuous variables were evaluated using t-test. Variables that were not normally distributed were evaluated using the nonparametric Mann-Whitney U test. Differences in categorical variables were evaluated using Fisher exact test or the Pearson chi-square test for more than two groups. Survival analysis was performed using the Kaplan-Meier method. Comparison of survival between the two groups was performed using the log-rank test. AEs in the less invasive group were compared with those for the conventional group for events in which the definitions were equivalent. All continuous data are summarized as mean ± standard deviation (SD). All comparisons were 2-sided with the level of significance set at P<0.05. Statistical analyses were performed using SPSS 20.0 (IBM SPSS Statistics, IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
Baseline characteristics were typical as of patients with end-stage HF implanted with LVADs (Table 1). These characteristics were similar between groups. Fifty-nine point four percent of all patients had idiopathic dilated cardiomyopathy (DCM), whereas 40.6% had ischemic cardiomyopathy (ICM). Patients were predominantly males with normal body mass index. Mean preoperative ejections fractions, measured by transthoracic echocardiography, averaged 17% (group A) to 18% (group B). Preoperative extracorporeal membrane oxygenation (ECMO) support was performed in 20 group A patients and in 11 group B patients.
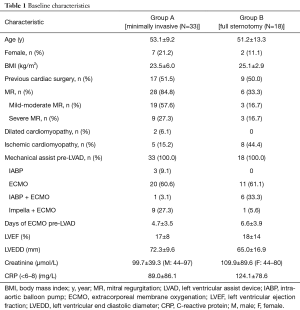
Full table
Outcomes
Total ICU stay was 16±18.2 days in group A and 18.2±12.9 days in group B (P=0.347). Total hospital stay was 31±68.8 days in group A and 36.0±45.6 days in group B (P=0.347). The duration of postoperative ECMO treatment was higher in group B (4.8±5.7 days) compared to group A (3.5±4.7 days). The most common causes of death were sepsis (36.4%), multi organ failure (27.3%) and RHF (18.2%).
Overall Kaplan-Meier survival (Table 2) was 69.7% in group A vs. 50.0% in group B at 3 and 12 months (P=0.191; log-rank test).

Full table
A comparison of AEs between groups is shown in Table 3. There were reductions or favourable trends in AEs in group A compared to group B in most major categories, including RHF, bleeding requiring surgery, infections, respiratory failure, renal failure and hepatic dysfunction. Stroke rate was comparable in both groups. The composite endpoint major adverse cardiac events (MACE) (RHF, respiratory failure, sepsis, renal dysfunction and significant bleeding) was 78.6% in group A vs. 94.4% of group B patients (P=0.178). There was only one group B patient who required pump exchange due to thrombosis during the follow-up period.
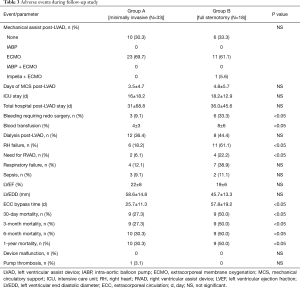
Full table
Discussion
Minimally invasive approaches in cardiac surgery have resulted in considerably reduced surgical trauma, leading to reduced rates of complications such as postoperative bleeding, postoperative pain and respiratory failure (12,13,20,21). Given the fact that the minimally invasive approaches for valve implantations were described to be beneficial regarding reduction of trauma to the patient and another given fact that the size of recently available LVADs became smaller and smaller during the past years, we developed a novel less traumatic LVAD implantation technique at our institution in Hannover, Germany consisting of a left-sided anterolateral thoracotomy and an upper J-shaped hemisternotomy (so called “Hannover-VAD-technique”) (16). The current study reveals the feasibility of this approach in critically ill patients requiring destination therapy and shows trends towards reduced rates of AEs and trends towards better long-term survival. This approach has several additional advantages compared to the full sternotomy conventional implantation approach that is already investigated in larger and longer term studies. First, right heart function is better protected using this technique; this is mainly due to two reasons: (I) to sew the apical sewing ring to the LV apex, the heart remains in its original position and has not to be lifted out of its anatomical position, thereby avoiding compressing the RV, as well as kinking of the right ventricular outflow tract (RVOT); (II) the pericardium remains largely intact, thereby avoiding overdilation of the RV through preserving the natural constraints of the right ventricle, which is hemodynamically very important following initiation of LVAD start. In clinical practice RV impairment is commonly managed by increased inotropic support. Our data suggest that the intra- as well as postoperative hemodynamic stability as significantly higher with minimal invasive surgery compared to standard surgery, as there was a lower incidence of RHF and a lower need for right ventricular assist devices (RVADs). Intraoperative weaning from extracorporeal circulation (ECC) was easier and patients were hemodynamically more stable when operated with a less invasive approach. This was also reflected in the low percentage of RV failure, shorter ECC bypass times and also the postoperative time on ECMO trended to be shorter as in thoracotomy patients. A second important benefit of the “Hannover-VAD-technique” is a lower incidence of reoperations due to postoperative bleeding, since lengths of incisions, surgical trauma as well as wound surface were considerably reduced in this group. Using our technique, it was possible to perform important surgical steps like the sewing ring suture off-pump, without being fully heparinised, thereby saving a huge amount of bypass time. In addition to the reduced incision size, this contributes to the observed decrease in blood loss, reduced need for blood transfusion and reduced rate of RHF. Importantly, the incidence of bleeding-related-surgery in the conventional sternotomy group was comparable to previously described studies (4).
Another potential advantage is, that due to the reduced surgical trauma after minimally invasive LVAD implantation, future redo operations (e.g., cardiac transplantation) might become technically facilitated and less risky as tissue adhesions remain reduced. Although our follow-up did not reveal a statistically significant difference in mortality (due to the low number of patients in the study), the Kaplan-Meier survival curves diverge during the first 3 months in favour of the minimal invasive approach. This early postoperative divergence reflects survival benefits directly related to the surgical technique including reduced surgery-related morbidity. However, prospective multi-centre studies in a larger number of patients are required to finally confirm advantages of this surgical approach in all aspects discussed above.
Despite the potential advantages, there are potential limitations associated with the minimal invasive approach. First, due to smaller incisions, the visibility of the whole heart is limited. Additionally, the estimation of the exact length of the outflow graft length is more difficult due to the limited view.
At this time, the latest publications do not recommend the immediate use of long-term VADs in INTERMACS 1 patients because of its high mortality. Thus, the use of short-term mechanical circulatory support enables hemodynamic stabilization. However, even if recompensation by this strategy is achieved, there remains an increased operative risk in these patients compared to INTERMACS 3 patients. The emergence of less invasive LVAD surgery enables to decrease mortality improving early outcomes in critically ill patients.
Study limitations
The results of this study need to be viewed in the context of several limitations. First, this was not a prospectively randomized study. All patients were operated by the same surgical team that also made the decision of which technique was used for implantation. Baseline characteristics did not significantly differ between both collectives. As the purpose of the study was to investigate safety, and not superiority of less invasive LVAD implantation in destination therapy patients, our data set the stage for future prospective multi-center studies.
Second, AEs were reported by treating physicians, were not monitored for completeness and were not adjudicated by an objective clinical events committee. While this did not affect the primary endpoint (survival), we cannot exclude under-reporting of other AEs.
Finally, this was a single-centre study performed at a highly experienced VAD centre. Since this is the first reported experience with this novel technique in INTERMACS 1 patients, the study was not performed on a multicentre level. Thus, a higher number of patients would have increased the statistical power of the study. However, as worldwide more and more sites gain experience applying less invasive surgery, there is a huge likelihood that a multicentre study will be feasible in the future.
Conclusions
Our data show that implantation of a miniaturized continuous flow LVAD through a minimally invasive thoracotomy approach is safe, feasible and associated with several benefits, including protection of the right ventricle, lower incidence of postoperative bleeding, reduced re-operations for bleeding and lower mortality. This minimally invasive approach reduces postoperative complications and improves the prognosis of INTERMACS 1 patients. Further studies are needed to confirm the results of this study.
Acknowledgements
JD Schmitto and G Dogan received research grants from Medtronic.
Footnote
Conflicts of Interest: JD Schmitto and G Dogan are consultants of Medtronic; LC Napp received lecture honoraria from Abiomed and Maquet. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local ethical committee of the Hannover Medical School. Patients gave informed consent to use their data in this retrospective analysis.
References
- Mathers CD, Boerma T, Ma Fat D. Global and regional causes of death. Br Med Bull 2009;92:7-32. [Crossref] [PubMed]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [Crossref] [PubMed]
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [Crossref] [PubMed]
- Strueber M, O'Driscoll G, Jansz P, et al. Multicenter evaluation of an intrapericardial left ventricular assist system. J Am Coll Cardiol 2011;57:1375-82. [Crossref] [PubMed]
- Dutt DP, Pinney SP. Clinical variability within the INTERMACS 1 profile: implications for treatment options. Curr Opin Cardiol 2014;29:244-9. [Crossref] [PubMed]
- Yang JA, Kato TS, Shulman BP, et al. Liver dysfunction as a predictor of outcomes in patients with advanced heart failure requiring ventricular assist device support: Use of the Model of End-stage Liver Disease (MELD) and MELD eXcluding INR (MELD-XI) scoring system. J Heart Lung Transplant 2012;31:601-10. [Crossref] [PubMed]
- Alba AC, Rao V, Ivanov J, et al. Usefulness of the INTERMACS scale to predict outcomes after mechanical assist device implantation. J Heart Lung Transplant 2009;28:827-33. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Avsar M, et al. Minimally-invasive LVAD Implantation: State of the Art. Curr Cardiol Rev 2015;11:246-51. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant 2014;33:555-64. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Long-term mechanical circulatory support (destination therapy): on track to compete with heart transplantation? J Thorac Cardiovasc Surg 2012;144:584-603; discussion 597-8. [Crossref] [PubMed]
- Riebandt J, Haberl T, Mahr S, et al. Preoperative patient optimization using extracorporeal life support improves outcomes of INTERMACS Level I patients receiving a permanent ventricular assist device. Eur J Cardiothorac Surg 2014;46:486-92; discussion 492. [Crossref] [PubMed]
- Cohn LH. Minimally invasive aortic valve surgery: technical considerations and results with the parasternal approach. J Card Surg 1998;13:302-5. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-invasive valve surgery. J Am Coll Cardiol 2010;56:455-62. [Crossref] [PubMed]
- Meyer AL, Malehsa D, Bara C, et al. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circ Heart Fail 2010;3:675-81. [Crossref] [PubMed]
- Schmitto JD, Avsar M, Haverich A. Increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:1463-4. [Crossref] [PubMed]
- Schmitto JD, Molitoris U, Haverich A, et al. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg 2012;143:511-3. [Crossref] [PubMed]
- Joyce DL, Conte JV, Russell SD, et al. Disparities in access to left ventricular assist device therapy. J Surg Res 2009;152:111-7. [Crossref] [PubMed]
- Bunte MC, Blackstone EH, Thuita L, et al. Major Bleeding During HeartMate II Support. J Am Coll Cardiol 2013;62:2188-96. [Crossref] [PubMed]
- Schmitto JD, Rojas SV, Hanke JS, et al. Minimally invasive left ventricular assist device explantation after cardiac recovery: surgical technical considerations. Artif Organs 2014;38:507-10. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Past, present, and future of minimally invasive mitral valve surgery. J Heart Valve Dis 2011;20:493-8. [PubMed]
- Schmitto JD, Mohr FW, Cohn LH. Minimally invasive aortic valve replacement: how does this perform in high-risk patients? Curr Opin Cardiol 2011;26:118-22. [Crossref] [PubMed]


