Early surgical treatment in patients with pulmonary embolism and thrombus-in-transit
Introduction
The presence of thrombi in right heart chambers can be a consequence of venous thromboembolism (VTE) or it can develop in situ as a consequence of cardiac conditions. Thrombus in transit (TT) or floating thrombus is defined as a thrombus temporarily located in the right heart chambers on its way to the pulmonary artery (PA). It is, therefore, a serious manifestation of VTE and with very high frequency (more than 90%) is associated with deep vein thrombosis (DVT) or pulmonary embolism (PE) (1). Given the high mortality rate without treatment (90%) and being largely precocious (in the first 24 hours), close monitoring in critical units and urgent treatment are warranted (including systemic fibrinolysis or surgical embolectomy in addition to conventional anticoagulation) (2,3). TT is an uncommon manifestation of symptomatic PE, with an approximate frequency of 4% (4-6). Currently, there is not enough evidence about the best treatment option, and recommendations are based on conclusions obtained from case series.
The objective of this work is to communicate our experience of early surgical management of patients with TT and PE in a Spanish tertiary hospital.
Methods
During the 2015–2017 period, 9 patients were assessed for early surgical thrombectomy. Patients were included if they had an acute symptomatic episode of PE and an imaging test [computed tomography pulmonary angiography (CTPA) or transthoracic echocardiogram (TTE)] showing right-heart thrombi, not adhered to walls. Four patients were dismissed (2 had a diagnosis of Behcet’s disease and received immunosuppressants and 2 due to elevated surgical risk). One patient was initially enrolled for early surgical thrombectomy but the thrombus migrated to the pulmonary arteries and, therefore, fibrinolytic therapy was administered. Finally, 4 patients underwent early surgical thrombectomy after signing informed consent.
The following variables were collected: age, sex, history of VTE, risk factors for VTE, symptoms at diagnosis (chest pain, syncope, dyspnea, signs of DVT), findings of Doppler ultrasound of the lower limbs, TTE and CT angiography findings, blood pressure (BP) and heart rate (HR) at diagnosis, D-dimer, troponin, Nt-proBNP, Pulmonary Embolism Severity Index (PESI) score, early mortality in the first 24 hours and first 30 days, clinical evolution and findings in the TTE at follow-up, and choice of anticoagulant treatment after surgery. Anticoagulant therapy with unfractionated heparin (UFH) was initiated after diagnosis and urgent cardiac thrombectomy with careful extraction of the thrombi were performed in all cases. In three of the patients, vasoactive drug infusion was required due to perioperative instability. Anticoagulation was continued with UFH and subsequently replaced by low molecular weight heparin and acenocoumarol.
Ethics approval was not required because all the patients were treated according to the current recommendations. Participants signed an informed consent before surgery and they also gave informed consent to publish his/her information.
Results
The sample included 3 men and 1 woman. The average age was 49.7 years (Table 1). The initial symptom was dyspnea (100%), signs of DVT (50%), chest pain (50%) and syncope (25%). The diagnosis of PE was made by angio-CT and the TT was found in TTE in all cases. TTE was performed prior to angio-CT in 1 patient. Two of the patients had provoking factors for VTE and the other two patients had a history of prior DVT. Surgical treatment consisted in atriectomy and manual extraction of the thrombus and/or trunk pulmonary opening and thrombi aspiration and it was performed in all patients in the first 24 hours after diagnosis, together with administration of subsequent UFH in all patients and vasoactive drugs in 3 of the patients in the perioperative period (one prior to surgery and two after surgery). All had right ventricular (RV) dysfunction at the time of diagnosis. The average value of D-dimer was 3,975 pg /mL, troponin levels were measured in three patients, being elevated in one of them. The average PESI score was 90 (range between 56 and 159). All patients presented a favorable clinical evolution (without dyspnea on exertion or chronic thromboembolic pulmonary hypertension), with complete recovery of RV function in two of them and residual dysfunction in the other 2. All survived at 30 days and at a mean follow-up of 15 months (range between 3 months and 26 months) with no hemorrhagic complications or recurrences of VTE (Table 2). Anticoagulant therapy was maintained indefinitely in all cases.
Full table

Full table
Patient 1 was a 56-year-old man with a history of diabetes, dyslipidemia and left popliteal DVT 8 years ago who was treated with enoxaparin for one month, with posterior postthrombotic syndrome. He went to the emergency department (ED) due to syncope and sudden onset of dyspnea, with a 15-day history of edema in right lower limb (RLL). On arrival at the ED BP was 107/84 mmHg, HR 120 bpm, oxygen saturation (SaO2) 96%. Angio-CT was performed showing bilateral PE with extensive filling defects in the bifurcation of the pulmonary trunk, main, lobar and segmental arteries (Figure 1). TTE showed a thrombus in the right atrium (RA) and RV, in addition to slight dilatation of the RV. Urgent thrombectomy was performed with RA opening and manual extraction of thrombi in right heart chambers, superior and inferior vena cava and pulmonary trunk opening with extraction of thrombi in it and in both main pulmonary arteries. During follow-up TTE was normal and thrombophilia study was performed, finding heterozygous mutation in 1691G>A of the F5 gene (factor V Leiden), and heterozygous mutation in 677C>T of the MTHFR gene without associated hyperhomocysteinemia.
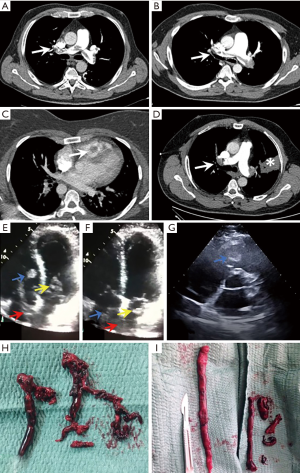
Patient 2 was a 46-year-old man with a history of obesity, central hypothyroidism and mixed anxiety-depressive disorder. He presented a prolonged hospital admission in an intensive care unit (ICU) 45 days before the current episode due to heat stroke with acute renal failure and severe hyponatremia. 3 weeks after discharge, he presented pleuritic chest pain and cough of 48 hours of evolution. Physical examination at ED included BP 122/70 mmHg, HR 107 bpm, respiratory rate (RR) 25 bpm, SaO2 95% and hypoventilation on the left lung base at pulmonary auscultation. The chest radiograph showed alveolar consolidation in the lingula. Angio-CT was performed, evidencing bilateral PE with involvement of the pulmonary trunk, main, lobar and segmental arteries of both lungs, with pulmonary infarction in lingula (Figure 1). TTE showed dilated RV with indirect data of pulmonary hypertension, together with a large thrombus in RA that entered into the left atrium (LA) through a patent foramen ovale (PFO) and that was introduced in both ventricles in diastole (Figure 1). Given these findings and the risk of paradoxical embolism, urgent thrombectomy was performed, with RA opening and thrombus removal, FPO closure and pulmonary trunk opening with manual removal of the thrombus (Figure 1). The thrombophilia study and the TTE at 6 months were normal.
Patient 3 was a 38-year-old woman with a history of prior spontaneous abortion, DVT during pregnancy 4 years before treated with anticoagulation until one month after delivery and a family history of VTE. She went to the ED due to progressive dyspnea on exertion and dry cough in the last 3 months. She also presented self-limited episodes of chest tightness, palpitations and fever up to 39 °C. On arrival upon ED, BP was 124/75 mmHg, HR 66 bpm, RR 26 bpm and SaO2 92%. TTE demonstrated the presence of a large thrombus in the RV (Figure 1), with dilatation and severe RV dysfunction. CT angiography was performed with a diagnosis of bilateral PE with filling defects in both lobar and segmental arteries and thrombus in RV (Figure 1). Urgent surgery was performed with RA opening and manual thrombi removal. Also pulmonary trunk was opened but no thrombi were found. The thrombophilia study was normal.
Patient 4 was a 59-year-old man with no prior medical history. He went to the ED for a 3-day history of progressive dyspnea on exertion with minimal effort and a 20-day history of edema in the RLL after a 10-hour plane trip. On physical examination BP was 135/65 mmHg, HR 130 bpm, SaO2 89%. Chest X-ray revealed a pulmonary consolidation in the left upper lobe and the EKG presented paroxysms of atrial fibrillation with rapid ventricular response. He was admitted to the ICU. CT angiography was performed showing bilateral PE with filling defects in the trunk, both main, lobar, segmental and subsegmental pulmonary arteries (Figure 1) and an area of pulmonary infarction with alveolar hemorrhage in left upper lobe. TTE showed thrombus in RA and inferior vena cava with protrusion to RV, which also presented severe dysfunction. Subsequently, the patient presented hemodynamic instability. Emergent surgical embolectomy was performed with pulmonary trunk opening and aspiration of thrombi in the pulmonary trunk, main and lobar arteries. TTE performed a week later showed slight RV dilatation. Clinical evolution was favorable 3 months after the intervention.
Discussion
Definition and epidemiology
The presence of thrombi in right heart chambers can be a consequence of VTE or it can develop in situ as a consequence of cardiac conditions. This differentiation is of great importance considering that they have different treatment and prognosis (1). TT or floating thrombus is defined as thrombus temporarily located in the right heart chambers on its way to the PA. It is, therefore, a serious manifestation of VTE and with very high frequency (more than 90%) is associated with DVT or PE (1). Therefore, the presence of a TT confirms the diagnosis of PE and implies the urge of immediate treatment without the need to perform additional diagnostic tests (2). There have been described three types of right heart thrombus using the TTE: type A thrombi are the most common and tend to be large and free floating masses with a high propensity for distal embolization; type B thrombi are small immobile right chamber clots attached to the walls originated in situ; finally, type C thrombi are rare and have great mobility mimicking atrial myxomas (3). TT is an uncommon manifestation of symptomatic PE, with an approximate frequency of 4% (4-6).
Prognosis
Though coexisting right heart thrombi (RHT) do not commonly occur in patients who have acute symptomatic PE, studies have validated RHT as predictor of poor outcome (7). Despite anticoagulation, the mortality rate has been reported high (23.2–44.7%), mainly in the first 24 hours (1-3,8,9). In a recent meta-analysis that included 15,220 patients with acute symptomatic PE, patients with concomitant echocardiography-detectable RHT had a 3-fold increased risk of short-term death compared to patients without RHT (6). Similar results were found in a study of patients with PE from the Registro Informatizado de la Enfermedad TromboEmbólica (RIETE) registry that included 12,441 patients with PE, of which 325 had RHT (10). Severe hypoxemia and the occurrence of cardiac arrest were significantly related to in-hospital mortality in patients with acute PE and concomitant TT (1). For these reasons, an early diagnosis and treatment is essential (3,11). However, some studies show that clinical prognostic scores (i.e., PESI, simplified PESI) have demonstrated an excellent accuracy for the identification of PE patients at low-risk of short-term complications and showed that coexisting TT in normotensive PE patients did not lead to a higher all-cause mortality. That notwithstanding, their data suggested an increased mortality risk for patients with TT and RV dysfunction (4,6). Conversely, prognosis is usually good after discharge (1).
Diagnosis
Most of TT are detected by TTE in patients diagnosed of acute PE or suspected of having it. Therefore, TTE is an important tool to its early recognition (1,5,10). In case of doubt, it would be necessary to perform a transesophageal echocardiogram, which is also useful to detect thrombi in the PA and the coexistence of PFO (1). Presence of PFO should be taken into consideration as it is a source of paradoxical embolus (emboli originated within the venous system, which pass through a PFO and enter the systemic circulation) and can be a site for the clot to lodge. Some authors favor surgical removal of these thrombi with simultaneous pulmonary embolectomy, due to the impending risk for dislodgement which may either lead to massive PE or paradoxical systemic embolism (5). Despite its prognostic importance, current guidelines do not propose that echocardiography is performed routinely in all patients with acute PE, or in all patients with a non-low-risk PESI assessment. However, they encourage assessment of right ventricular function by echocardiography and/or measurement of cardiac biomarkers if, following clinical assessment, there is uncertainty about whether patients require more intensive monitoring or should receive thrombolytic therapy (6,12).
Treatment
Current guidelines suggest that patients with acute PE with hypotension (i.e., systolic BP <90 mmHg for 15 min) and without high bleeding risk are treated with thrombolytic therapy. Development of hypotension suggests that thrombolytic therapy has become indicated. Deterioration that has not resulted in hypotension may also prompt the use of thrombolytic therapy. For example, there may be a progressive increase in HR, a decrease in systolic BP (which remains >90 mmHg), an increase in jugular venous pressure, worsening gas exchange, signs of shock (e.g., cold sweaty skin, reduced urine output, confusion), progressive right heart dysfunction on echocardiography, or an increase in cardiac biomarkers (12). However, thrombolytic therapy is not warranted in most cases in abscense of hypotension (5,12-14). Few studies, only case series and retrospective studies, have compared thrombolysis with surgical therapy in acute PE and recommended to consider surgical pulmonary embolectomy in case of hypotension and contraindication for thrombolysis or life-threatening situations like failure of thrombolysis, RV failure, cardiogenic shock and TT (3,12,15,16). According to current European Society of Cardiology guidelines, in patients with TT, the therapeutic benefits of thrombolysis remain controversial (16). Surgical embolectomy is warranted in some cases of TT, for example, those with the coexistence of PFO. Optimal treatment for acute PE and concomitant TT is not defined currently due to the absence of randomized controlled trials and it must be considered on a case-by-case basis by multidisciplinary teams after a risk-benefit assessment (4-6,16). Patients receiving no therapy had a 90–100% mortality rate (2,3,8). Furthermore, mortality rate is high (8,9) (21.1%) (2) within the first 24 hours, which warrants an immediate treatment (3,16). Thus, inotropic support with catecholamine infusion should be prepared immediately after the diagnosis of TT and administered as soon as either the patient’s BP falls below 100 mmHg or cardiogenic shock is suspected. It may be preferable to admit the patients to an ICU, because sudden death is a risk and because mechanical ventilation may be required (1). Different therapeutic approaches have been reported for acute PE with concomitant TT: anticoagulation with UFH, systemic thrombolysis with recombinant tissue plasminogen activator (rTPA), surgical embolectomy with exploration of the right chambers and the pulmonary arteries under full cardiopulmonary bypass, and endovascular thrombectomy (1,5). Systemic thrombolysis and surgical embolectomy showed a higher probability of survival (81.5% and 70.45% respectively) in comparison with anticoagulation (47.7%) (2,3,8). Nevertheless, there are contradictory data regarding the TT management; a recent study that included patients with acute PE associated with TT from the RIETE registry showed that there were no significant differences for mortality and bleeding between reperfusion therapy (i.e., thrombolysis, surgery) and anticoagulation (7). Regarding the superiority of thrombolysis or surgical thrombectomy, both are effective strategies, with a slightly increased probability of survival with thrombolysis in some studies. They have, however, been plagued by selection bias, small numbers and a lack of comparable groups. A well-designed prospective, randomized trial is needed to determine the optimal treatment of acute PE and concomitant TT (3,8). In our series, although three patients had cardiogenic shock during the perioperatory period, only one presented it before the surgical treatment and the others had high risk of developing it. Subsequently, emergent surgical embolectomy was performed with RA and/or pulmonary trunk opening and manual removal of thrombi with an excellent result and a favorable clinical evolution (without dyspnea on exertion or chronic thromboembolic pulmonary hypertension). Finally, the duration of anticoagulation in patients with acute PE and concomitant TT is not defined. According to current guidelines, the duration of anticoagulation is mainly based on the identification of reversible provoking factors (12,16). In our series, 50% of patients had transient provoking factors and the other 50% of patients had a history of VTE. In all cases the decisive factor to decide the duration of anticoagulation was the severity of the episode. Thus, anticoagulation with acenocoumarol was maintained indefinitely with a periodic assessment of the bleeding risk.
Conclusions
Thrombus-in-transit is an uncommon and serious manifestation of VTE. The high early mortality rate warrants a rapid and effective treatment. Surgical embolectomy in patients with PE and concomitant thrombus-in-transit can be an effective treatment in selected patients, although the current evidence to support this approach is not definitive.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Hospital General Universitario Gregorio Marañón. Participants signed an informed consent before surgery and they also gave informed consent to publish his/her information.
References
- Chartier L, Béra J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999;99:2779-83. [Crossref] [PubMed]
- Arboine-Aguirre L, Figueroa-Calderón E, Ramírez-Rivera A, et al. Thrombus in transit and submassive pulmonary thromboembolism succesfully treated with tenecteplase. Gac Med Mex 2017;153:129-33. [PubMed]
- Athappan G, Sengodan P, Chacko P, et al. Comparative efficacy of different modalities for treatment of right heart thrombi in transit: a pooled analysis. Vasc Med 2015;20:131-8. [Crossref] [PubMed]
- Koć M, Kostrubiec M, Elikowski W, et al. Outcome of patients with right heart thrombi: the Right Heart Thrombi European Registry. Eur Respir J 2016;47:869-75. [Crossref] [PubMed]
- Agarwal V, Nalluri N, Shariff MA, et al. Large embolus in transit - an unresolved therapeutic dilemma (case report and review of literature). Heart Lung 2014;43:152-4. [Crossref] [PubMed]
- Barrios D, Rosa-Salazar V, Morillo R, et al. Prognostic significance of right heart thrombi in patients with acute symptomatic pulmonary embolism: systematic review and meta-analysis. Chest 2017;151:409-16. [Crossref] [PubMed]
- Barrios D, Chavant J, Jiménez D, et al. Treatment of Right Heart Thrombi Associated with Acute Pulmonary Embolism. Am J Med 2017;130:588-95. [Crossref] [PubMed]
- Rose PS, Punjabi NM, Pearse DB. Treatment of right heart thromboemboli. Chest 2002;121:806-14. [Crossref] [PubMed]
- Torbicki A, Galié N, Covezzoli A, et al. Right heart thrombi in pulmonary embolism: results from the International Cooperative Pulmonary Embolism Registry. J Am Coll Cardiol 2003;41:2245-51. [Crossref] [PubMed]
- Barrios D, Rosa-Salazar V, Jiménez D, et al. Right heart thrombi in pulmonary embolism. Eur Respir J 2016;48:1377-85. [Crossref] [PubMed]
- Bodian M, Ba FG, Jobe M, et al. Fatal evolution of a huge right atrial free-floating thrombus. Clin Case Rep 2013;1:63-5. [Crossref] [PubMed]
- Kearon C, Akl EA, Omelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and expert panel report. Chest 2016;149:315-52. [Crossref] [PubMed]
- Konstantinides SV, Barco S, Lankeit M, et al. Management of pulmonary embolism: an update. J Am Coll Cardiol 2016;67:976-90. [Crossref] [PubMed]
- Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J 2015;36:605-14. [Crossref] [PubMed]
- Sareyyupoglu B, Greason K, Suri R, et al. A more aggressive approach to emergency embolectomy for acute pulmonary embolism. Mayo Clin Proc 2010;85:785-90. [Crossref] [PubMed]
- Konstantinides SV, Torbicki A, Agnelli G, et al. Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35: 3033-69, 3069a-3069k.


