What is the role of wedge resection for T1a lung cancer?
Introduction
Since 1995, lobar resection became the standard of care for medically fit patients with early stage lung cancer. This was based on the results of the randomized trial (1) conducted by the lung cancer study group (LCSG) between 1982 and 1988, where 247 patients clinically staged as T1N0 were intra-operatively randomly assigned to either lobar or sub-lobar resection. It is noteworthy that the only required modalities for clinical staging were bronchoscopy and plain chest radiography. Lobar resections were performed in 125 patients while sub-lobar resections were done in 122 (wedges 33%, segments 67%). The investigators found a statistically significant tripling in loco-regional recurrence (LR) after limited resection but no difference between the two arms of the trial in systemic recurrence. Although both overall survival and cancer specific survival favored lobectomy, neither achieved statistical significance. A particularly troubling result of the LSCG trial was that wedge resections were associated with a 2-fold increase in LR compared to segmentectomy and a 4-fold increase compared to lobectomy. These findings essentially substantiated the notion that wedge resection is an oncologically unsound treatment and is an inferior modality of sub-lobar resection and should be restricted to patients with profound limitations in cardiopulmonary reserve in whom segmentectomy is not an option.
Wedge resection revisited
Although the results of the LCSG trial have dominated the field over the past three decades, the trial suffers from important flaws in both design and methodology that limit its relevance to patients with lung cancer diagnosed in an era of significant advances in imaging modalities and surgical strategies. For example, modern imaging modalities such as high resolution computed tomography (CT) and positron emission tomography (PET) imaging have totally replaced plain chest radiography as staging modalities and permit a more precise determination of the clinical stage of the disease. Furthermore, with the more broad utilization of chest CT in general, and the recent approval of lung cancer screening by low dose CT (2), patients are being diagnosed with smaller, earlier stage lung cancers. The increased detection of smaller more precisely staged tumors combined with the rising segment of the population that is elderly with limited cardiopulmonary reserve has renewed interest in sub-lobar resection including wedge resection as either a definitive therapeutic strategy or as a compromise approach in patients with poor performance status. The interest in wedge resections is also to some extent further fueled by the emergence and increased utilization of competing technologies of local control such as stereotactic radiation or percutaneous and trans-bronchial ablative techniques. Although the results of the LCSG still cast a long shadow over the soundness of wedge resection as a cancer operation, the previous discussion almost compels us to ask the question again: is there a role for wedge resection in often small early stage lung cancers?
The conflicting evidence for limited resection
Many studies of various designs have been performed since the LCSG evaluated oncologic outcomes following sublobar resection (SLR). Unfortunately, these studies have often resulted in divergent conclusions. In the years since the LCSG several case series compared lobectomy to limited resection including wedge resection. The majority of these case series—especially those from North American and European centers—concluded that limited resection, particularly wedge resection, were associated with higher rates of local recurrence and inferior survival to lobectomy (3-6). The majority of these case series were confounded by variable study design, strong selection bias, heterogeneity in tumor size, and lack of adequate information on the extent of nodal staging.
There are at least seven published meta-analyses (7-13) (Table 1) of randomized trials and case series comparing lobectomy with sub-lobar resections. Again, these meta-analyses produced conflicting results with hazard ratios favoring lobectomy in some and limited resection in others. Taioli et al. (13) concluded that the great majority of studies are sufficiently heterogeneous precluding calculation of reliable meta-estimates.
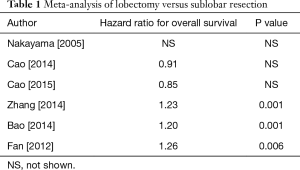
Full table
Several studies took advantage of the large number of patients in national databases in North America to compare lobar and sub-lobar resection. For example, at least nine analyses (14-22) of the Surveillance, Epidemiology, and End Results (SEER) data-base compared lobectomy with sub-lobar resections (predominantly wedge resections) over the last decade (Table 2). Remarkably, five studies showed superiority of lobectomy and four showed no difference in outcomes. How could nine studies of a single database result in such divergent results? It is our view that national data-bases with their large number of patients can usefully show temporal variations in disease prevalence or treatment strategies; however, they generally lack the required granularity in clinical variables that are often involved in selecting one type of treatment over another. An interesting analysis of the SEER data-base by Yendamuri and colleagues in 2013 (18) (Figure 1), shows that the superiority of lobar resection over sub-lobar resection diminishes with each passing decade with equivalency between sub-lobar resection and lobectomy in the outcomes of patients treated between 2005 and 2008. A reasonable interpretation of these results is that the improvement in clinical staging modalities over time may be largely responsible for the reduction in differences in survival based on the extent of resection.
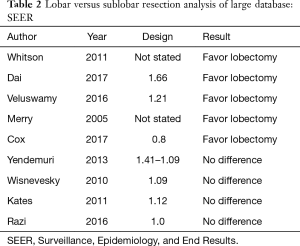
Full table
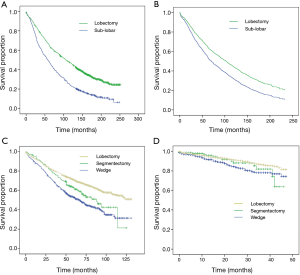
Is wedge resection equivalent to lobectomy?
Most of the recent American series comparing SLR to lobectomy have unsurprisingly consisted of patients with marginal cardiopulmonary reserve. And while these series have included both wedge resections and segmentectomy, a majority of SLR resections in American series have been performed by wedge resection. However, there is some recent data comparing wedge resection and lobectomy in medically fit patients. In 2014, Altorki et al. (22) published a study comparing outcomes following lobectomy and SLR in patients registered in the International Early Lung Cancer Action Program. Patients with lung cancer detected with LDCT in this large international screening study and who had clinical IA lung cancers were treated with either lobectomy (N=294) or SLR (N=53). The majority of the SLR group underwent wedge resection (N=37, 69.8%). The vast majority of tumors were clinical T1a (88.2%). Importantly, there were no significant differences in comorbidities between the two groups. Ten-year survival estimates for lobectomy and SLR were identical: 86% for SLR versus 85% for lobectomy. While the patients in this study were a very select group—patients diagnosed through lung cancer screening—the overall survival of 85% or greater for all patients and the equivalence between SLR and lobectomy were remarkable. However, it should be emphasized that these results were derived from a retrospective analysis of a large screening data base and that patients were not randomly assigned to the extent of parenchymal resection.
Is wedge resection equivalent to segmentectomy
As noted above, most SLR resections are performed in medically compromised patients. However, one longstanding controversy is whether anatomic segmentectomy improves oncologic results compared to wedge resection. The published data are once again marred by inherent overt and latent biases precluding meaningful conclusions. However, analysis of the results of a large randomized trial may provide interesting insights into the limited resection debate. ACOSOG Z4032 (23) evaluated the role of brachytherapy in reducing local recurrence following sub-lobar resection. High risk patients with clinical stage I non-small cell lung cancer were randomly assigned to sub-lobar resection with and without brachytherapy. The trial showed no difference in either the primary endpoint of local recurrence or in the secondary endpoint of overall survival. Wedge resections were done in 155 out of 222 patients or 70% of all patients on trial. Local recurrence occurred in 12% of all patients and in 15% in patients who had a wedge resection. Following wedge resection three year local recurrence free survival was 67% and overall 3-year survival was 70% which were similar to three year outcomes for the whole cohort. While the trial was not designed specifically to compare wedge and segmentectomy, it is a contemporary prospective randomized trial that lends support to the notion that wedge resection and anatomical segmentectomy maybe equivalent methods of sub-lobar resection.
In 2016 Altorki et al. (24) reported a retrospective analysis of an institutional database including patients with clinical T1a lung cancer treated with either wedge resection or segmentectomy. Predictably, the majority of these patients were offered SLR due to significant cardiopulmonary morbidity (76% of wedge resection and 62% of segmentectomy). There was no difference in comorbidity or pulmonary function between the groups. Patients undergoing segmentectomy compared to wedge resection had clinically (1.7 vs. 1.5 cm, P=0.002) and pathologically (1.7 vs. 1.5 cm, P=0.002) larger tumors. Although resection free margins were achieved in 99% of all patients, segmentectomy resulted in greater median resection margins compared to wedge resection (1.5 vs. 1.0 cm, P=0.001). Additionally, lymph node sampling was markedly higher than in prior studies (95% following segmentectomy and 71% following wedge resection). Despite a greater resection margin after segmentectomy, there was no difference in either local recurrence or five year overall survival between segmentectomy and wedge resection. The authors therefore concluded that for clinical T1a tumors, wedge resection maybe oncologically equivalent to anatomic segmentectomy.
Status of contemporary randomized trials
With the conflicting results surrounding sub-lobar resection presented in this review and in light of the limitations of the LCSG trial, ultimately the question of the oncologic soundness of wedge resection will be answered in the setting of large randomized trials. There are two recently closed randomized trials from Japan and North America that will provide insight into the role of sub-lobar resection. The Japanese trial (JCOG 0802) randomized patients with small peripheral tumors (2 cm or less) to either lobectomy or anatomical segmentectomy. No wedge resections were allowed in JCOG 0802. The phase III American study—CALGB 140503—is designed as a non-inferiority trial to compare lobectomy with sub-lobar resection for solid tumors sized 2 cm or less located in the periphery of the lung. Sub-lobar resection in this trial includes either wedge resection or segmentectomy. While neither of these trials will answer which sublobar technique (wedge resection versus anatomic segmentectomy) is optimal—the Japanese trial only included AS, and the North American trial was not adequately powered to compare WR versus AS—these two large randomized trials will hopefully provide insight into whether lobectomy and SLR are equivalent in the medically fit patients. While the final conclusions of these trials will not be reported for several years, it is anticipated that these two trials will set the standard of care for decades.
Summary
In conclusion, while wedge resection has been considered “inferior” as a cancer operation for nearly 20 years, the more contemporary literature is conflicting and inconclusive. There have been several recent studies even suggesting an equivalence between wedge resection and lobectomy. Differences in overall survival between wedge and lobectomy appear largely related to inadequate lymph node sampling at the time of wedge resection, and this is a particularly important point given the surprisingly low rates of lymph node sampling in the earlier published studies. Regardless, there is sufficient controversy that, in the absence of modern prospective trials, overly dogmatic statements regarding the inferiority of wedge resections should be avoided. Instead when wedge resection is required, the focus should be on the quality of the wedge resection—with the surgeon routinely performing hilar and mediastinal lymph node sampling and ensuring as much as possible a wide resection margin. Performed well, wedge resection maybe an appropriate surgical option. Recently concluded randomized trials will likely set the standard of care for decades to come. As we await the results of these trials, the immediate future must be one of personalized surgical approaches—approaches which take into consideration the individual patient’s characteristics, tumor imaging characteristics, and implications for quality of life and surgical recovery. These considerations, along with shared decision making with the patient, will ultimately become standard of care for the treatment of early stage lung cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: Presented at the 1st International Conference Sublobar Resections for Lung Cancer Paris, France.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg 1997;113:691-8; discussion 698-700. [Crossref] [PubMed]
- Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. Ann Thorac Surg 2011;92:1819-23; discussion 1824-5.
- Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg 1994;107:1087-93; discussion 1093-4. [PubMed]
- Sienel W, Dango S, Kirschbaum A, et al. Sublobar resections in stage IA non-small cell lung cancer: segmentectomies result in significantly better cancer-related survival than wedge resections. Eur J Cardiothorac Surg 2008;33:728-34. [Crossref] [PubMed]
- Cao C, Gupta S, Chandrakumar D, et al. Meta-analysis of intentional sublobar resections versus lobectomy for early stage non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:134-41. [PubMed]
- Zhang Y, Sun Y, Wang R, et al. Meta-analysis of lobectomy, segmentectomy, and wedge resection for stage I non-small cell lung cancer. J Surg Oncol 2015;111:334-40. [Crossref] [PubMed]
- Nakamura H, Kawasaki N, Taguchi M, et al. Survival following lobectomy vs limited resection for stage I lung cancer: a meta-analysis. Br J Cancer 2005;92:1033-7. [Crossref] [PubMed]
- Bao F, Ye P, Yang Y, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta-analysis Eur J Cardiothorac Surg 2014;46:1-7. [Crossref] [PubMed]
- Fan J, Wang L, Jiang GN, et al. Sublobectomy versus lobectomy for stage I non-small-cell lung cancer, a meta-analysis of published studies. Ann Surg Oncol 2012;19:661-8. [Crossref] [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Taioli E, Yip R, Olkin I, et al. Survival after sublobar resection for early-stage lung cancer: methodological obstacles in comparing the efficacy to lobectomy. J Thorac Oncol 2016;11:400-6. [Crossref] [PubMed]
- Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg 2011;92:1943-50. [Crossref] [PubMed]
- Dai C, Shen J, Ren Y, et al. Choice of surgical procedure for patients with non-small-cell lung cancer</1⁄4 1 cm or > 1 to 2 cm among lobectomy, segmentectomy, and wedge resection: a population-based study. J Clin Oncol 2016;34:3175-82. [Crossref] [PubMed]
- Veluswamy RR, Ezer N, Mhango G, et al. Limited Resection Versus Lobectomy for Older Patients With Early-Stage Lung Cancer: Impact of Histology. J Clin Oncol 2015;33:3447-53. [Crossref] [PubMed]
- Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest 2005;128:237-45. [Crossref] [PubMed]
- Yendamuri S, Sharma R, Demmy M, et al. Temporal trends in outcomes following sublobar and lobar resections for small (</=2 cm) non-small cell lung cancers-a Surveillance Epidemiology End Results database analysis. J Surg Res 2013;183:27-32. [Crossref] [PubMed]
- Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg 2010;251:550-4. [Crossref] [PubMed]
- Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer <=1 cm in size: a review of SEER data. Chest 2011;139:491-6. [Crossref] [PubMed]
- Razi SS, Daskalaki D, Burack J. Current trends in lung resection for T1a non-small cell lung cancer: is lobectomy still the answer? J Thorac Dis 2017;9:E164-E165. [Crossref] [PubMed]
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62; discussion 762-4. [Crossref] [PubMed]
- Fernando HC, Santos RS, Benfield JR, et al. Lobar and sublobar resection with and without brachytherapy for small stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2005;129:261-7. [Crossref] [PubMed]
- Altorki NK, Kamel MK, Narula N, et al. Anatomical segmentectomy and wedge resections are associated with comparable outcomes for patients with small ct1n0 non-small cell lung cancer. J Thorac Oncol 2016;11:1984-92. [Crossref] [PubMed]


