The alteration of T790M between 19 del and L858R in NSCLC in the course of EGFR-TKIs therapy: a literature-based pooled analysis
Introduction
Major advances in the treatment of non-small cell lung cancer (NSCLC) in the last decade have arisen from the recognition that specific genetic alterations define subsets of NSCLC (1). Activating mutations in the gene which encode the epidermal growth factor receptor (EGFR) protein are the most extensively studied and present in 10–20% of Caucasian NSCLC cases, and 30–50% of Asian NSCLC cases (2). The most therapeutic-relevant mutations are 19 del (exon 19 deletions) and L858R mutation. Together, the two sensitive mutations occupy over 80% of EGFR mutations in NSCLC (3).
EGFR-tyrosine kinase inhibitors (EGFR-TKIs) have proven fantastic response rates and more acceptable toxicity profiles compared to traditional standard chemotherapy in patients with advanced NSCLC harboring sensitive EGFR mutations (exons 19 and 21) (4). Unfortunately, most patients with NSCLC develop acquired resistance to TKIs and experience disease progression within 12 months after the initiation of TKI therapy (5). The secondary mutation in exon 20, T790M, detected in approximately 50% of re-biopsy samples after TKI therapy, is regarded as the most common cause of acquired resistance to TKIs (6). Exon 20 insertions account for 4–9% of all EGFR mutant lung tumors before receiving TKIs, which have been shown to confer resistance to subsequent TKI therapy (7). However, if T790M mutations are selected from de novo T790M mutation in TKI-naïve patients as a minor clone or are acquired during TKIs therapy remains debated (8). Recently, the FLURUA study indicated the third generation TKI, Osimertinib, showed efficacy superior to that of standard first-generation EGFR-TKIs in the first-line treatment of EGFR mutation-positive advanced NSCLC, with a similar safety profile and lower rates of serious adverse events.
A previous meta-analysis suggested that T790M mutation is more inclined to coexist with L858R than with 19 del in NSCLC patients pre-TKIs [odds ratio (OR), 1.65; 95% confidence intervals (95% CIs), 1.17 to 2.32], which might be one of the reasons that 19 del was related to a better outcome than L858R with TKIs (9). However, TKIs might alter this status due to differing sensitivities of these mutations to drugs and dynamic changes associated with secondary mutations or sub-clone selection (10). To study the mechanism of resistance to TKIs in NSCLC patients and thus to develop novel treatment and rational therapy strategies, information on tumor characteristics throughout the course of the disease and therapy is essential. We sought to compare the prevalence of T790M between 19 del and L858R upon acquired resistance to TKIs by assembling all existing data.
Methods
Literature search and selection
A systematic and comprehensive literature search of online databases PubMed, Web of Science, Medline and Cochrane library was performed to identify observational studies and RCTs conducted before July 2017 that examined studies on T790M mutation for NSCLC patients.
Several terms and their variants were used, including EGFR, T790M, lung cancer, NSCLC, TKIs, and resistance. The references of identified papers, previously published systematic reviews, and meta-analysis were inspected to identify studies not included by the initial search.
We reviewed all searched results according to the PRISMA statement (11). The selection of original studies was based on the process of viewing titles, abstracts and full papers. The inclusion criteria were as follows: (I) studies that focused on patients with NSCLC; (II) data regarding 19 del and L858R with or without T790M mutations after TKIs treatment. Review articles, abstracts, case reports, editorials, and letters were excluded.
Data extraction and quality assessment
All data were recorded independently by two researchers (HR Liang and DF Chen). Any conflict was resolved by the third researcher (WH Liang). For the selected studies, information on all available variables was extracted and entered into a Microsoft Excel database. The numbers of 19 del and L858R with or without T790M mutations were extracted to compute the co-existing rate. Patients with co-existing L858R and 19 del mutations were excluded. Original data from previous meta-analysis were also extracted and re-analyzed to explore the mechanism of drug resistance during TKI therapy (9). We performed subgroup analyses according to the study design, population race, and sample types. Quality of each paper was assessed using the Joanna Briggs Institute Prevalence Critical Appraisal Tool (12). Any disagreement was resolved via discussion amongst the authors.
Statistical analysis
OR with its 95% CIs were computed to compare the prevalence of T790M between 19 del and L858R. We used Cochran’s I2 test and X2 to examine the heterogeneity among included studies. Statistical heterogeneity among studies was defined as I2 statistic greater than 50% (13). Random effects model was preferred owing to the heterogeneity among studies (14). Single-arm and re-analysis were also performed to evaluate the co-existence of T790M with 19 del or L858R in our study and previous meta-analysis. Statistical significance was settled as two-sided P<0.05. The analysis was conducted with STATA 12.0.
Results
Study selection and quality assessment
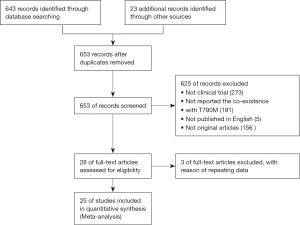
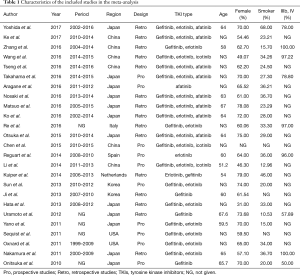
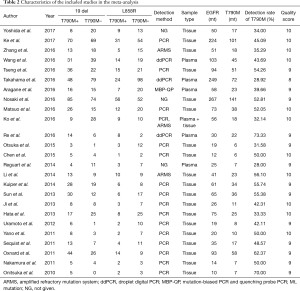
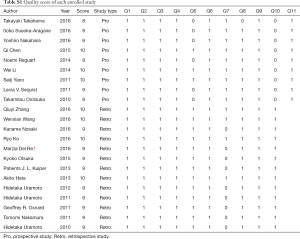
A total of 643 records were screened from the previously mentioned online databases with the search cutoff of June 30th 2017. After excluding duplicates, a manual search and inspection of the reference lists and existing reviews identified 23 additional relevant studies. Further review according to inclusion criteria led to the final selection of the 25 papers considered in this analysis (Figure 1) (10,15-38). The contextual details and the results of the quality assessment of each study were summarized in Tables 1,2. The detailed quality score of each enrolled study is shown in the Table S1 (quality score of each enrolled study).
Full table
Full table
Full table
The studies were conducted in 7 different countries and the period ranged from 2010 to 2017. Ten articles were prospective studies and the others were retrospective. A total of 25 studies involving a total of 1,770 patients were included. The overall T790M detection rate of post-TKIs was 45.25%. All studies gained 8 to 10 stars in study quality assessment on a scale of 0 to 10.
Primary outcome
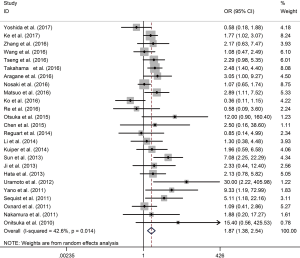
In single-arm synthesis, the co-existence rate of T790M was 53% (95% CI, 47% to 59%) and 36% (95% CI, 30% to 42%) in 19 del and L858R, respectively. Post-TKIs T790M was significantly more frequently co-existing with 19 del than L858R mutated patients (OR 1.87; 95% CI, 1.38 to 2.54; P<0.001). Statistical heterogeneity was moderate among studies (I2 =42.6; P=0.014) (Figure 2).
Subgroup analysis
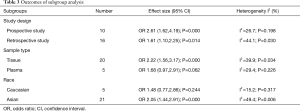
We performed subgroup analysis stratified by the study design, population race and sample types. The relative effects were consistently significant in most subgroups except in Caucasians (OR 1.48; P=0.244) and in studies using plasma (OR 1.68; P=0.062), but the trend remained the same. No significant heterogeneity was found among studies in any of the subgroups (Table 3).
Full table
Comparison with the previous meta-analysis
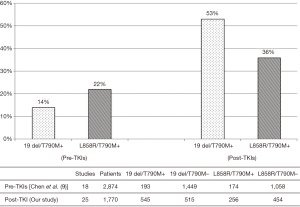
We re-analyzed the previous meta-analysis and made a single-arm meta-analysis (9), finding that the pooled rate of pre-TKIs T790M was 14% (95% CI, 10% to 17%) and 22% (95% CI, 16% to 27%) in 19 del and L858R respectively. Pre-TKIs T790M was significantly less frequently co-existing with 19 del than L858R mutated patients (OR 0.59; 95% CI, 0.44 to 0.80; P<0.001) with no heterogeneity (I2 =0; P=0.788), while the co-existing rate of T790M mutation became 53% (95% CI, 47% to 59%) and 36% (95% CI, 30% to 42%) in 19 del and L858R after TKI resistance in our pooled analysis. The increase of the T790M co-existing rate was 2.79-fold in 19 del but only 0.63-fold in L858R (Figure 3).
Discussion
Newly acquired resistance in sensitive EGFR mutation positive NSCLC patients after first-generation TKI therapy is a tough problem. The T790M mutation can be detected in nearly 50% patients at time of acquiring TKI-resistance (39). The results of a previous meta-analysis reported that pre-TKIs T790M is less frequent in patients harboring 19 del compared to those carrying the L858R mutation (14% vs. 22%; OR 0.59; P<0.001) (9). The trend of our study differed from the previous study in that T790M was more frequent in exon 19 deletion than in L858R among patients with acquired resistance to TKIs (53% vs. 36%; OR 1.87; P<0.001). In subgroup analyses by study design, sample size and race, we found results were similar to overall analyses.
Much debate exists regarding the “selection” or “acquisition” of the T790M mutation formation process. This mutation was initially thought to be acquired only after exposure to TKIs because T790M was lacking in pre-progression samples. However, T790M has been identified in TKI treatment-naive NSCLC samples using standard sequencing methods, which indicated T790M is a common mutation in some TKI-naive tumors, and this alteration are picked by following TKI treatment (40). One of the interpretations to explain the “reversal” of T790M prevalence between 19 del and L858R in the course of TKI therapy might be patients with 19 del mutation are more sensitive to TKIs (41), thus the sub-clone of T790M mutation in 19 del patients are more likely to be selected and enriched.
The median survival of late stage NSCLC patients is less than 2 years after the emergence of T790M mutation (42). Recently, third-generation TKIs, such as AZD9291 (Osimertinib), have emerged as a potential therapeutic option to fight against the EGFR T790M-positive tumors (43). However, there is a great number of patients harboring the T790M mutation that are either unavailable to re-biopsy or of which the mutation has not been detected and thus unable to receive these treatments. Besides, the sensitivity of plasma detection is not satisfactory, thus these patients who are detected as negative T790M mutation via plasma may be also potentially benefited from Osimertinib. Our study suggested that patients harboring the 19 del mutation may be more likely to gain profit from Osimertinib than those with L858R if they attempt treatment when it is not clearly indicated.
Even so, there is still a subset of patients who will receive repeat first-generation TKIs as salvage treatment after initial failure. To date, no satisfying biomarkers have been found to indicate which patients may receive benefit from the continuation of first-generation TKI therapy. One study found that T790M positive patients had better prognosis with both longer PFS and OS than those without the mutation, and, therefore, claimed patients with acquired T790M mutation at the time of progression may benefit more from TKI re-challenge (30). But another study reported that survival outcomes of secondary TKIs did not differ significantly between T790M-positive and T790M-negative groups; this indicated that T790M might not predict the clinical outcomes in first-generation TKI re-challenge (16). These findings suggested there is heterogeneity in predictive effect on TKI therapy and a difference in the prognosis of 19 del and L858R patients. TKIs are more effective in 19 del patients, and our pooled analysis observed the acquired resistance as a result of the T790M mutation is more likely attributed to 19 del than L858R. Thus, we speculate that it might be the proportion of L858R-T790M mutation, not the T790M mutation alone, that could predict the outcome of first generation TKI re-challenge and suppose the continuation of first-generation TKIs in patients harboring 19 del might be less recommended.
In addition, different prognostic effects were observed in pre- and post-TKI T790M positive NSCLC patients. A meta–analysis demonstrated that pre-TKI T790M positive NSCLC patients were associated with a worse outcome in either PFS or OS, while newly acquired T790M mutation after TKI therapy seemed to be a good prognostic factor (44). Another meta-analysis also linked the pre-TKI T790M mutation with a negative impact on the PFS for NSCLC patients (45). The worse outcomes correlated with the assumption that pre-TKI T790M mutation might be a result of the decreased sensitivity of T790M positive cell to TKIs, while the better prognosis of T790M positive patients after TKI therapy could be explained by the indolent biologic behaviors of T790M positive cells (46). There is evidence that patients who had exon 19 deletions had statistically significant longer PFS than those with L858R(47). Our study suggested that the proportion of patients with coexisting 19del and T790M mutations might obtain better survival.
We acknowledge several limitations to our study. First, not all of the included studies were prospective randomized comparisons, which increase the risk of potential selection and reporting bias. Second, though moderate, heterogeneity among studies (I2 =42.6; P=0.014) still existed. Different baseline characteristics, differing sensitivities of detection methods, and small populations in each study might explain the heterogeneity. Third, the data we used are based on the published literature rather than primary data as we were unable obtain unpublished data. Last, though we observed more T790M mutations co-existing with 19 del post-TKIs, we do not know the state of T790M abundance in 19 del and L858R.
Conclusions
Unlike the situation of de novo T790M, an opposite trend that T790M was more frequent in exon 19 deletion than in L858R among TKI acquired resistance patients was observed. The resistance lead by T790M mutation is more likely attributed to 19 del than L858R, which indicated that re-challenge of first-generation TKIs may be less recommended in patients harboring 19 del. Our study also suggested that patients who harbored 19 del mutation may be more likely to gain profit from Osimertinib than those with L858R if they attempt treatment when it is not clearly indicated. In all, our results encouraged developing detection or treatment strategies in L858R and 19 del patients, respectively, for the specific resistance mechanism.
Acknowledgements
We thank Lindsey Hamblin for assistance with the language revision.
Funding: This work was supported by the following funding:
Chinese National Natural Science Foundation (Grant No. 81501996); Guangdong Doctoral Launching Program (Grant No. 2014A030310460); Doctoral Launching Program of Guangzhou Medical University (Grant No. 2014C27);
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Berge EM, Doebele RC. Targeted therapies in non-small cell lung cancer: emerging oncogene targets following the success of epidermal growth factor receptor. Semin Oncol 2014;41:110-25. [Crossref] [PubMed]
- Oxnard GR, Lo PC, Nishino M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol 2013;8:179-84. [Crossref] [PubMed]
- Ahsan A. Mechanisms of Resistance to EGFR Tyrosine Kinase Inhibitors and Therapeutic Approaches: An Update. Adv Exp Med Biol 2016;893:137-53. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Ercan D, Choi HG, Yun CH, et al. EGFR Mutations and Resistance to Irreversible Pyrimidine-Based EGFR Inhibitors. Clin Cancer Res 2015;21:3913-23. [Crossref] [PubMed]
- Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014;11:473-81. [Crossref] [PubMed]
- Inukai M, Toyooka S, Ito S, et al. Presence of epidermal growth factor receptor gene T790M mutation as a minor clone in non-small cell lung cancer. Cancer Res 2006;66:7854-8. [Crossref] [PubMed]
- Leone A. Highly sensitive detection of EGFR T790M mutation in pre-TKI specimens of EGFR-mutated NSCLC: in cis, in trans, or a different clone? J Thorac Oncol 2013;8:e26-7. [Crossref] [PubMed]
- Chen LY, Molina-Vila MA, Ruan SY, et al. Coexistence of EGFR T790M mutation and common activating mutations in pretreatment non-small cell lung cancer: A systematic review and meta-analysis. Lung Cancer 2016;94:46-53. [Crossref] [PubMed]
- Nosaki K, Satouchi M, Kurata T, et al. Re-biopsy status among non-small cell lung cancer patients in Japan: A retrospective study. Lung Cancer 2016;101:1-8. [Crossref] [PubMed]
- Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011;39:91-2. [Crossref] [PubMed]
- Munn Z, Moola S, Riitano D, et al. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag 2014;3:123-8. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Uramoto H, Yamada T, Yano S, et al. Prognostic value of acquired resistance-related molecules in Japanese patients with NSCLC treated with an EGFR-TKI. Anticancer Res 2012;32:3785-90. [PubMed]
- Zhang Q, Ke E, Niu F, et al. The role of T790M mutation in EGFR-TKI re-challenge for patients with EGFR-mutant advanced lung adenocarcinoma. Oncotarget 2017;8:4994-5002. [PubMed]
- Yoshida T, Kuroda H, Oya Y, et al. Clinical outcomes of platinum-based chemotherapy according to T790M mutation status in EGFR-positive non-small cell lung cancer patients after initial EGFR-TKI failure. Lung Cancer 2017;109:89-91. [Crossref] [PubMed]
- Yano S, Yamada T, Takeuchi S, et al. Hepatocyte growth factor expression in EGFR mutant lung cancer with intrinsic and acquired resistance to tyrosine kinase inhibitors in a Japanese cohort. J Thorac Oncol 2011;6:2011-7. [Crossref] [PubMed]
- Wang W, Song Z, Zhang Y. A Comparison of ddPCR and ARMS for detecting EGFR T790M status in ctDNA from advanced NSCLC patients with acquired EGFR-TKI resistance. Cancer Med 2017;6:154-62. [Crossref] [PubMed]
- Takahama T, Sakai K, Takeda M, et al. Detection of the T790M mutation of EGFR in plasma of advanced non-small cell lung cancer patients with acquired resistance to tyrosine kinase inhibitors (West Japan oncology group 8014LTR study). Oncotarget 2016;7:58492-9. [Crossref] [PubMed]
- Sun JM, Ahn MJ, Choi YL, et al. Clinical implications of T790M mutation in patients with acquired resistance to EGFR tyrosine kinase inhibitors. Lung Cancer 2013;82:294-8. [Crossref] [PubMed]
- Sueoka-Aragane N, Katakami N, Satouchi M, et al. Monitoring EGFR T790M with plasma DNA from lung cancer patients in a prospective observational study. Cancer Sci 2016;107:162-7. [Crossref] [PubMed]
- Reguart N, Rosell R, Cardenal F, et al. Phase I/II trial of vorinostat (SAHA) and erlotinib for non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations after erlotinib progression. Lung Cancer 2014;84:161-7. [Crossref] [PubMed]
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22. [Crossref] [PubMed]
- Otsuka K, Hata A, Takeshita J, et al. EGFR-TKI rechallenge with bevacizumab in EGFR-mutant non-small cell lung cancer. Cancer Chemother Pharmacol 2015;76:835-41. [Crossref] [PubMed]
- Onitsuka T, Uramoto H, Nose N, et al. Acquired resistance to gefitinib: the contribution of mechanisms other than the T790M, MET, and HGF status. Lung Cancer 2010;68:198-203. [Crossref] [PubMed]
- Tseng JS, Su KY, Yang TY, et al. The emergence of T790M mutation in EGFR-mutant lung adenocarcinoma patients having a history of acquired resistance to EGFR-TKI: focus on rebiopsy timing and long-term existence of T790M. Oncotarget 2016;7:48059-69. [Crossref] [PubMed]
- Nakamura T, Sueoka-Aragane N, Iwanaga K, et al. A noninvasive system for monitoring resistance to epidermal growth factor receptor tyrosine kinase inhibitors with plasma DNA. J Thorac Oncol 2011;6:1639-48. [Crossref] [PubMed]
- Matsuo N, Azuma K, Sakai K, et al. Association of EGFR Exon 19 Deletion and EGFR-TKI Treatment Duration with Frequency of T790M Mutation in EGFR-Mutant Lung Cancer Patients. Sci Rep 2016;6:36458. [Crossref] [PubMed]
- Li W, Ren S, Li J, et al. T790M mutation is associated with better efficacy of treatment beyond progression with EGFR-TKI in advanced NSCLC patients. Lung Cancer 2014;84:295-300. [Crossref] [PubMed]
- Kuiper JL, Heideman DA, Thunnissen E, et al. Incidence of T790M mutation in (sequential) rebiopsies in EGFR-mutated NSCLC-patients. Lung Cancer 2014;85:19-24. [Crossref] [PubMed]
- Ko R, Kenmotsu H, Serizawa M, et al. Frequency of EGFR T790M mutation and multimutational profiles of rebiopsy samples from non-small cell lung cancer developing acquired resistance to EGFR tyrosine kinase inhibitors in Japanese patients. BMC Cancer 2016;16:864. [Crossref] [PubMed]
- Ji W, Choi CM, Rho JK, et al. Mechanisms of acquired resistance to EGFR-tyrosine kinase inhibitor in Korean patients with lung cancer. BMC Cancer 2013;13:606. [Crossref] [PubMed]
- Hata A, Katakami N, Yoshioka H, et al. Rebiopsy of non-small cell lung cancer patients with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor: Comparison between T790M mutation-positive and mutation-negative populations. Cancer 2013;119:4325-32. [Crossref] [PubMed]
- Del Re M, Tiseo M, Bordi P, et al. Contribution of KRAS mutations and c.2369C > T (p.T790M) EGFR to acquired resistance to EGFR-TKIs in EGFR mutant NSCLC: a study on circulating tumor DNA. Oncotarget 2017;8:13611-9. [Crossref] [PubMed]
- Chen Q, Quan Q, Ding L, et al. Continuation of epidermal growth factor receptor tyrosine kinase inhibitor treatment prolongs disease control in non-small-cell lung cancers with acquired resistance to EGFR tyrosine kinase inhibitors. Oncotarget 2015;6:24904-11. [PubMed]
- Ke EE, Zhou Q, Zhang QY, et al. A higher proportion of the EGFR T790M mutation may contribute to the better survival of patients with exon 19 deletions compared with those with L858R. J Thorac Oncol 2017;12:1368-75. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008;105:2070-5. [Crossref] [PubMed]
- Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 2008;359:366-77. [Crossref] [PubMed]
- Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 2006;12:839-44. [Crossref] [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046-61. [Crossref] [PubMed]
- Liu Y, Sun L, Xiong ZC, et al. Meta-analysis of the impact of de novo and acquired EGFR T790M mutations on the prognosis of patients with non-small cell lung cancer receiving EGFR-TKIs. Onco Targets Ther 2017;10:2267-79. [Crossref] [PubMed]
- Ding D, Yu Y, Li Z, et al. The predictive role of pretreatment epidermal growth factor receptor T790M mutation on the progression-free survival of tyrosine-kinase inhibitor-treated non-small cell lung cancer patients: a meta-analysis. Onco Targets Ther 2014;7:387-93. [PubMed]
- Chmielecki J, Foo J, Oxnard GR, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med 2011;3:90ra59. [Crossref] [PubMed]
- Zhang Y, Sheng J, Kang S, et al. Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis. PLoS One 2014;9:e107161. [Crossref] [PubMed]


