A new tool for an explantation strategy of HeartMate 3™ left ventricular assist device
Introduction
Due to consistently improved technology and miniaturization, left ventricular assist devices (LVADs) have become a promising therapeutic alternative to cardiac transplantation in patients with end-stage heart failure (1,2). LVADs are implanted as bridge to transplant, bridge to recovery and as destination therapy. The potential of cardiac recovery after mechanical support has been demonstrated in several previous studies and is the most desirable goal in the treatment of end-stage heart failure with LVADs, allowing device-explantation and avoiding cardiac transplantation and lifelong mechanical support, with reduced complications (3,4). Currently, depending on the cardiac pathology, only few patients can be weaned from ventricular assist devices. However, the growing number of patients undergoing LVAD implantation especially in earlier stages of congestive heart failure, new minimally invasive implantation techniques and a growing experience in the field of mechanical support could lead to an increase of cardiac recoveries.
Several explantation strategies for different LVADs have been described in existing literature. These include ventriculoplasty with removal of the sewing ring, or sewing ring preservation and occlusion of the cored ventriculotomy, each with or without the removal of the outflow graft (5,6).
Herein, we present a novel tool for an explantation strategy of the HeartMate 3™ (HM3, Abbott, North Chicago, IL, USA) LVAD (Figure 1). This tool has been invented by Croleon Innovation Labs Pvt. Ltd., India (patent pending; application number: 201721044329) and manufactured by INNOVO Solutions GmbH, Germany (Email: info@innovo-solutions.de) under license and provided to Hannover Medical School for experimentation. Another design was developed as an alternative, allowing mechanical attachment of the plug to the sewing ring (Figure 2).


Operative procedure
For the explantation of LVADs, a minimally invasive surgical approach via anterolateral thoracotomy has become the gold standard in our clinic, as it effectively reduces perioperative complications such as bleeding or right heart failure and minimizes the surgical trauma (7).
After the anterolateral thoracotomy, the pump housing and the outflow graft are dissected from adhesions. Guide wires are then placed percutaneously into the femoral vessels with subsequently cannulation as per Seldinger technique in order to apply cardiopulmonary bypass. The outflow graft is then divided and distally closed with a purse string-suture, remaining in situ. The LVAD inflow cannula is explanted out of the left ventricle after gradual activation of cardiopulmonary bypass. The left ventricle is thoroughly inspected for potential left ventricular thrombi and a custom-made mechanical plug is inserted into the ventricular coring and fixed onto the sewing ring with several sutures (Figure 3). The driveline is then completely removed percutaneously. After stepwise weaning from cardiopulmonary bypass, wounds are closed and the patient is admitted to the intensive care unit for further postoperative stabilization.
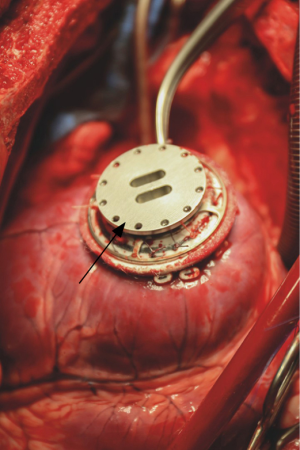
Discussion
Recovery of cardiac function is the holy grail in the treatment of congestive heart failure. Due to the growing number of patients undergoing LVAD implantation, there is an increasing number of patients with the potential of device explantation (8). Therefore, novel explantation techniques avoiding perioperative complications need to be established. So far, a mechanical plug for LVAD explantation has only been available for the HeartMate II (Abbott, North Chicago, IL, USA) and HVAD (HeartWare Inc., Framingham, MA, USA) (5,9). In this paper we present a novel custom-made tool for an explantation technique of the HeartMate 3™ LVAD.
One of the major advantages of the mechanical plug is that it can be implanted minimally invasively. Owing to the minimized surgical trauma, in complication rates including postoperative bleeding are reduced with consecutively lower need for transfusion products and reduced pain. Additionally, using this implantation technique, the pericardium stays intact which leads to avoidance of right heart failure, one of the major factors for mortality in patients undergoing cardiac surgery. In addition, the postoperative bleeding risk is significantly reduced as the wound surface is reduced and pericardial tamponades can not occur.
Additionally, the implantation of this plug allows not only for an off-pump explantation, but for a fast redo-implantation in case of current left ventricular failure, as the sewing ring is preserved. This also leads to an intact left ventricular geometry, so that the amount of myocardium contributing to left ventricular strokes can be kept at a maximum.
In contrast to implantation techniques with sewing ring removal, leaving the sewing ring attached, as in the abovementioned procedure enables the possibility of a quick LVAD re-implantation in the case of future cardiac impairment.
When using a mechanical plug for the occlusion of the ventricular coring, an artificial surface gets in contact with blood, leading to a potential risk for thrombi-formation, embolisation and the need for anticoagulation therapy. This could result in a higher occurrence of postoperative bleeding. However, a recent study for an explantation of the HeartMate 2™ shows, that after LVAD explantation without sewing ring removal using an individual designed mechanical plug, there is a formation of connective tissue covering the foreign surface. Regarding this, anticoagulant-therapy could be only necessary for an early postoperative stage, until the formation of connective tissue has taken place, which would lead to a dramatically reduction of potential bleeding risks.
Yet, the use of mechanical plugs has not become a clinical standard and as it is still not a CE-marked therapy and therefore off-label. When considering this approach, patients should be thoroughly informed about the advantages and disadvantages of this technique. Inconclusive cases should still be treated with standard approaches.
Conclusions
LVAD implantation is a promising therapeutic option for end-stage heart failure (10). In this article we present a novel tool and surgical technique is presented for the explantation of the HeartMate 3TM ventricular assist device in case of cardiac recovery. The custom-made mechanical plug (manufactured by: INNOVO Solutions GmbH, Germany; contact: info@innovo-solutions.de) fits into the sewing ring and seems to be a promising alternative to standard device-explantation procedures. Due to the lack of commercially available products, this custom manufactured implantable plug may be considered for use under compassionate use conditions.
Acknowledgements
None.
Footnote
Conflicts of Interest: Jan D. Schmitto and Guenes Dogan are consultants for Abbott. Other authors have no conflicts of interest to declare.
References
- Schmitto JD, Hanke JS, Rojas S V, et al. First implantation in man of a new magnetically levitated left ventricular assist device (HeartMate III). J Heart Lung Transplant 2015;34:858-60. [Crossref] [PubMed]
- Schmitto JD, Pya Y, Zimpfer D, et al. HeartMate 3 fully magnetically levitated left ventricular assist device for the treatment of advanced heart failure-CE Mark Study 2-Year Results. J Heart Lung Transplant 2017;36:S66. [Crossref]
- Birks EJ. Molecular changes after left ventricular assist device support for heart failure. Circ Res 2013;113:777-91. [Crossref] [PubMed]
- Hall JL, Fermin DR, Birks EJ, et al. Clinical, molecular, and genomic changes in response to a left ventricular assist device. J Am Coll Cardiol 2011;57:641-52. [Crossref] [PubMed]
- Schmitto JD, Rojas S V, Hanke JS, et al. Minimally invasive left ventricular assist device explantation after cardiac recovery: surgical technical considerations. Artif Organs 2014;38:507-10. [Crossref] [PubMed]
- Frazier OH, Baldwin ACW, Demirozu ZT, et al. Ventricular reconditioning and pump explantation in patients supported by continuous-flow left ventricular assist devices. J Heart Lung Transplant 2015;34:766-72. [Crossref] [PubMed]
- Schmitto JD, Deniz E, Rojas SV, et al. Minimally invasive implantation: the procedure of choice! Oper Tech Thorac Cardiovasc Surg 2016;21:65-78. [Crossref]
- Jakovljevic DG, Yacoub MH, Schueler S, et al. Left ventricular assist device as a bridge to recovery for patients with advanced heart failure. J Am Coll Cardiol 2017;69:1924-33. [Crossref] [PubMed]
- Potapov EV, Stepanenko A, Hennig E, et al. A titanium plug simplifies left ventricular assist device removal after myocardial recovery. J Heart Lung Transplant 2010;29:1316-7. [Crossref] [PubMed]
- Feldmann C, Chatterjee A, Haverich A, et al. Left Ventricular Assist Devices - A State of the Art Review. Adv Exp Med Biol 2018;1067:287-94. [Crossref] [PubMed]


