Effect of lornoxicam in lung inflammatory response syndrome after operations for cardiac surgery with cardiopulmonary bypass
Introduction
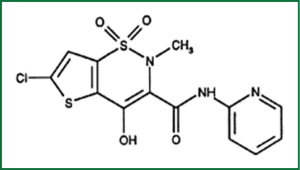
Lornoxicam known as chlortenoxicam is an NSAID and belongs to the category of oxicams. Its molecular weight is 371.81 and the colour yellow. Lornoxicam is a zwitterion at pH 2-5 and anion at pH ≥6.208. Lornoxicam has analgesic, antipyretic and anti-inflammatory action (Figure 1). The lornoxicam inhibits the action of cyclooxygenase enzyme and as result prevents the synthesis of prostaglandins some of which have harmful effects on the body. The linoleic acid of foods converted into phospholipids of the cell membrane, which under the influence of phospholipase A2 are converted into arachidonic acid, which essentially is the precursor of prostaglandins. Subsequently arachidonic acid undergoes oxidation and cyclization with the effect synthetase endoperoxide of prostaglandin, and the prostaglandins are produced PGG2, from which—again with the effect of the same enzyme—prostaglandins PGH2 are produced. The latter are precursors of various prostaglandins and thromboxanes. The lornoxicam acts by inhibiting the action of the enzyme cyclooxygenase (cyclization) and peroxidase (oxidation), an action which is characterized as “reversible” that lasts about 48 hours. As shown in Figure 1, arachidonic acid follows simultaneously another metabolic road where the influence of 5-lipoxygenase neutrophil is converted into leukotrienes. The synthesis of prostaglandins can be inhibited, except lornoxicam and other compounds, such as cortisol, aspirin and other NSAIDs. Major interest in the mechanism of action of lornoxicam presents, as it is shown in Figure 1, the participation of the enzyme cyclooxygenase (COX). There are two types of cyclooxygenase: COX-1 and COX-2. The COX-1 is located in the gastric mucosa, platelets, vascular endothelium and kidney. COX-2 is produced in response to the inflammatory reaction and therefore is found in monocytes and macrophages when they are activated by the activated platelet factor (PAF), interleukin-1 or lip-polysaccharide bacteria. Also, COX-2 is located in the smooth muscle cells, epithelial and endothelial cells and neurons. Many of the non-selective NSAIDs inhibit both types of cyclooxygenase, leading to undesirable side effects from the gastrointestinal tract after COX-1 is inhibited. Lornoxicam exhibits potent inhibitory activity in both isozymes (1).
The study was conducted in 14 patients who have been undergone elective coronary bypass surgery by extracorporeal circulation. All patients were informed before surgery for the purpose of study and having fully understood the investigation and possible complications of the additional intraoperative manipulations, and they gave written consent for their participation in the study.
All patients were examined clinically radio graphically and Spiro metric for foreclosure of pulmonary disease. From the study excluded patients:
- With a history of chronic obstructive pulmonary disease or asthma or other lung disease;
- With chronic atrial fibrillation;
- With a projected time AOC <30 min;
- Who have been undergone urgent myocardial reperfusion;
- With an ejection fraction of the left ventricle <30%;
- With a history of gastritis or peptic ulcer.
Seven patients (six males, one female) with a mean age 66.5±7.5 years constituted the control group (Group A) and they did not receive any substance.
Other seven patients (six males, one female) with a mean age 65.8±5.9 years constituted the study group of lornoxicam (group B). Patients were administered intravenous lornoxicam, 8 mg at induction of anesthesia and 8 mg immediately before the commencement of the EC. The formulation that had been used was Xefo® (Nycomed-Hellas).
Preparation of pharmaceutical agent
The lornoxicam (formulation Xefo) is in the form of water soluble powder, yellow color, in total vials containing 8 mg. The dissolution of the formulation is accomplished using a solvent (distilled water), which coexists in a separate ampoule 2 mL. The final solution is to be administered to 2 mL and the active substance (LNX) 8 mg.
Surgical technique
All the patients of the study had been undergone in coronary bypass under extracorporeal circulation. The deference of blood from the right atrium was performed using intracardiac catheter (venous cannulae) double layer. Arterial cannula EC was placed in the ascending aorta. The congestion of the heart (venting) was conducted by the pulmonary artery by inserting a special catheter. Membrane oxygenators were used (Dideco, Milano, Italy) and it was applied moderate hem dilution (Hct 22-25%). The EC was streamed, providing ≥2.4 lit/min/m2 slight systemic hypothermia (33-35 °C temperature bladder). Mean arterial pressure during EC was maintained at levels greater than 55 mmHg.
At first distal shunts were carried out by the grafts (venous and arterial). The central shunts of the grafts were carried out after the lifting of the blockade of the aorta, during the phase of myocardial reperfusion (reperfusion phase) after partial exclusion of the aorta with special atraumatic vascular clamp.
Protocol of cardioplegia
For myocardial protection during the ischemic period of the heart was applied normothermic hyperkalemic cardioplegia with a ratio of blood to cardioplegic solution: 1 lit blood/50 mL cardioplegic solution. The cardioplegic solution contained a total of 40 mEq K+ and 20.4 mEq Mg2+.
Initially it was granted a quantity of 1,200-1,500 mL blood cardioplegia to the root of the aorta, with flow injection 300-320 mL/min and injection pressure at the root of the aorta 120-140 mmHg to achieve cardiac asystole. During the exclusion of the aorta and after completion of each distal shunt was administered additional dose of cardioplegic solution (250-350 mL/min) at the root of the aorta. After the removal of AOC and during the conduct of central shunts it was awarded oxygenated blood through the vein grafts directly from the EC.
Protocol of anesthesia
The patients were assessed preoperatively on the day before surgery. As a premedication was administered diazepam 10 mg orally one hour before the transfer of the patient to the operating room. The anesthetic preparation included connection of patient to the monitor (Solar 8000, Marquette Medical Systems, Milwaukee, USA), placement of peripheral venous 16G, 20G arterial line in the radial or brachial artery and sensor placement BIS (BIS/XP, Aspect Medical Systems, USA).
The induction of anesthesia was by intravenous administration of midazolam (Dor-micum) dosed of 0.01-0.03 mg/kg, fentanyl citrate (Fentanyl) 1-2 mg/kg, and etomidate (Hypnomidate) 0.2 mg/kg and pre-oxygenation of the patient with oxygen mask. The laryngoscopy and tracheal intubation were made following the granting of pancuronium (Pavulon), 1-1.2 mg/kg and the patient was connected to the anesthesia machine (Julian, Draeger, Berlin, Germany) taking amount of mechanical ventilation (IPPV) that aimed at slightly hypocapnia, which was estimated by the values of expired dioxide and PaCO2 (33 mmHg < PaCO2 <37 mmHg). The maintenance of anesthesia was with propofol (Diprivan) in dosage 5-6 mg/kg/h, with continuous infusion pumps (Fresenius, Vial, Brezins, France) aimed at values of about 30-45 and BIS remifentanil hydrochloride (Ultiva) a dose of 20 mg/kg/h, while the maintenance of muscle relaxation with pancuronium continuous doses.
In patients the right jugular vein was catheterized and triaflos catheter and sheath 8.5 French were placed. The hemodynamic monitoring was completed by placing the pulmonary artery catheter (Oximetry TD catheter, Edwards Lifesciences, Berlin, Germany). Vital parameters of cardiac operating and hemodynamic that are considered are listed below:
- Monitor Marquette Solar 8000: electronic control cardioscopic of five efferent continuous analysis of space ST, automatic analysis based clusters reference ECG arrhythmia detection, continuous (bloody) blood pressure measurement, continuous measurement of pulmonary artery pressure, pulmonary artery pressure exclusion (PAOP);
- Oximetry TD catheter: cardiac output (CO), stroke volume (SV), mixed venous saturation (SvO2), systemic resistance and pulmonary circulation (SVR & PVR), index project pulse left and right ventricular (LVSWI & RVSWI).
Regarding respiratory (lung mechanics and sharing) it was controlled pulse oximetry (SpO2), the expired dioxide (EtCO2), pressures and flow expiratory airway (Paw & Faw), the forced expiratory volume and pulmonary compliance. In addition, patients were under continuously temperature control with special catheter bladder while blood gases, the acid-base balance, blood sugar and serum electrolytes and lactate concentration were monitored periodically. The gelling ability of the blood was checked periodically with time Activated Clotting Time (ACT).
Study design
In all patients in the study demographic data were recorded demographic data, aggravating factors and the data of coronary angiography. Also there were mapped the following stimulative factors: number of bypass grafts, total time EC, time AOC, total surgery time and the pressures in the pulmonary circulation (before sternotomy, before entering the extracorporeal circulation and at the end of surgery). In addition to each patient the following were applied:
They were determined: (I) the levels of cytokines IL-6, IL-8, TNF-a; (II) levels of ICAM 1, E-Selectin, P-Selectin; and (III) the levels of MMP-3.
The blood samples were from the femoral artery through a special catheter Ms-16G, which was placed immediately after general anesthesia before the beginning of the surgery.
Blood samples were taken: (I) immediately after anesthesia induction (Δ0); (II) prior to intravenous administration of heparin and the entry of extracorporeal circulation (Δ1); (III) immediately after the release of the EP (Δ2); (IV) immediately after the surgery—in ICU (Δ3); (V) immediately after non-cannulation of the patient (Δ4) averaged about five hours after the entry into the ICU and (VI) the morning of the first postoperative day (Δ5).
Microscopic study of lung tissue pieces
Lung biopsies were taken immediately after opening the sternum (B0) and just before the closure of sternotomy (B1). The obtained lung tissue pieces were placed in 10% formalin solution and were consigned to the Department of Pathology.
After appropriate treatment of historical pieces and hematoxylin-eosin staining, the study was under the microscope magnification ×200 and was focused on the count of eosinophils in the lung parenchyma, on the study of thickness of the alveoli capillary membrane, on searching interstitial edema and generally on the finding changes the structure of lung tissue.
Check the functionality of the heart and lungs in the ICU
The control functionality of the heart and lungs in the ICU was occurred in 2nd, 6th and 12th hour after the transfer, by identifying of the following parameters:
- Pulmonary pressures;
- Cardiac index (CI);
- Systemic vascular resistance index (SVRI);
- Pulmonary vascular resistance index (PVRI);
- Pointer left ventricular work (LVSWI);
- Right ventricular work index (RVSWI);
- Alveolar arterial oxygen difference D (A-a), according to the Eq. [1]:
- Pulmonary compliance, from the ones disclosed parameters of ventilator;
- Intrapulmonary shunt (Qs/Qt) based on the Eq. [2]:
[1] |
[2] |
where
(arterial blood)
(venous blood).
Statistical analysis
For the statistical analysis of the data used in statistical accounting package SPSS for Windows, version 12.0. The level of significance (significant level) for each test was set at P<0.05. To test the homogeneity of the two groups and the possibility of a dispute as to the average of the measurements of the variables of the two groups, the methodology is as follows.
- In the quantitative variables (age, body surface area, ejection fraction, peak expiratory capacity, expiratory volume in second indicator Tiff-eneau, number of bypass grafts, EC time, time AOC, time of surgery) was examined whether or not there was a significant difference between the average measurements for both patient groups. The homogeneity tests were performed using the Statistical T-test for the variables which followed the normal distribution and the Mann Whitney U test for those who did not follow a normal distribution. The control for adjusting or not observations was under the Shapiro-Wilk test.
- In the array variables (sorted by NYHA, sorted by angina CCS) the existence or not of significant difference in measurements was examined by Mann Whitney U test.
- In qualitative variables (sex, diabetes mellitus, hypertension, smoking) the control of homogeneity between the two groups was performed using statistical Fisher’s Exact test.
- In the case of repeated measurements for each variable (pulmonary artery pressure, alveolar-arterial oxygen difference, intrapulmonary shunt, cardiac index, systemic vascular resistance, pulmonary vascular resistance index, left ventricular work index, right ventricular work index, markers of inflammation blood) the test for statistical difference existence was under by univariate approach, as it gives more robust results when the number of patients is small, provided the regularity and uniformity of measurements. The first condition (regularity) was tested as the above with the Shapiro-Wilk test. Testing for homogeneity was done with Mauchly’s test of sphericity. If the test rejected the uniformity then the correction Huynth-Feldt has been applied which gave the corrected results. Simultaneously with repeat measurements the statistical power (power control) was determined. In cases that significant results or insignificant but with little effect were raised, additional investigation was conducted comparing all classes in pairs using control Bonferroni.
Results
Preoperative data
Demographic, clinical and laboratory features of the two groups of patients are presented in Table 1. The study of these characteristics showed that the two groups were comparable in terms of gender, age and body surface area. Incidences of diabetes, hypertension, and smoking rates did not differ significantly between the two groups. The distribution of patients in various stages of angina (classification CCS) and staging of heart failure NYHA did not differ between the two groups. Also there was no statistically significant difference in left ventricular function with regard to the determination of ejection fraction measurements and the spirometry tests between two groups of patients.
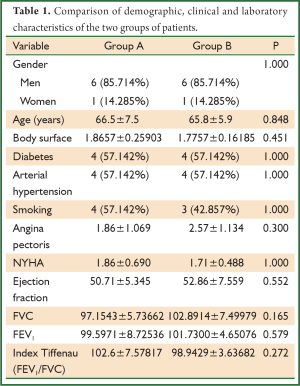
Full table
Intraoperative data
All patients after the removal of AOC and the onset of reperfusion phase showed sinus rhythm automatically. In one patient in the control group, due to sinus rhythm with low frequency (pulse <40/min) was applied temporary atrioventricular pacing. Low-dose of epinephrine (≤0.04 mg/kg/min) was administered in two patients in the control group and in a one patient from the group of lornoxicam.
The intraoperative patient characteristics of both groups are presented in Table 2. There was neither statistically significant difference between the two patient groups in the mean time EC (116.140±30.824 min in the control group vs. 101.430±34.097 min in group LNX), nor in the mean time AOC (68.000±22.758 min in the control group versus 54.000±25.222 min in group of Lor-noxikamis). Statistically significant difference between the two groups was not observed and the total time of surgery (4.500±0.408 h in the control group vs. 4.714±0.809 h in the lornoxicam group).
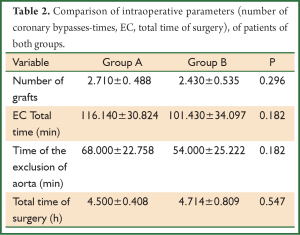
Full table
Regarding the intraoperative measurements of values of pulmonary artery pressure (systolic-diastolic) it was found that the average systolic pressure in the third measurement (end of surgery) was statistically significantly lower in the lornoxicam group compared to the control group (Table 3).
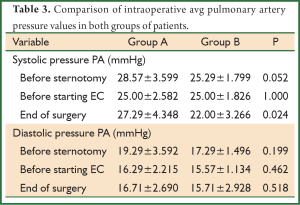
Full table
Data in ICU
All the patients in our study were incubated by a period of 4-6 hours after entering the ICU. During hospitalization in Unit they did not show any major complication. After intubation the minimum doses of adrenaline in the above patients they were gradually decreased and stopped. The ECG and cardiac enzymes in patients of both groups they did not provide any indicative evidence of intraoperative myocardial infarction and their stay in the ICU was 48 hours. Two patients in the control group and one patient in the lornoxicam group presented the morning of the second postoperative day atrial fibrillation with rapid ventricular response which was treated with intravenous amiodarone drip. The measured hemodynamic parameters of the two groups of patients were presented in Table 4, in which the mean values and the standard deviation of these are listed. It was not recorded any statistically significant difference between the two groups regarding the pulmonary artery pressures. Also it was not recorded any statistically significant difference between the two groups of patients in: (I) the cardiac index (CI); (II) the work of the left pulse (LVSWI) and right ventricular (RVSWI); and (III) the index of systemic (SVRI) and pulmonary vascular resistance (PVRI). The measured values of alveolar-arterial oxygen difference [D (A-a)], the intrapulmonary shunt (Qs/Qt) and lung compliance were not statistically significant.
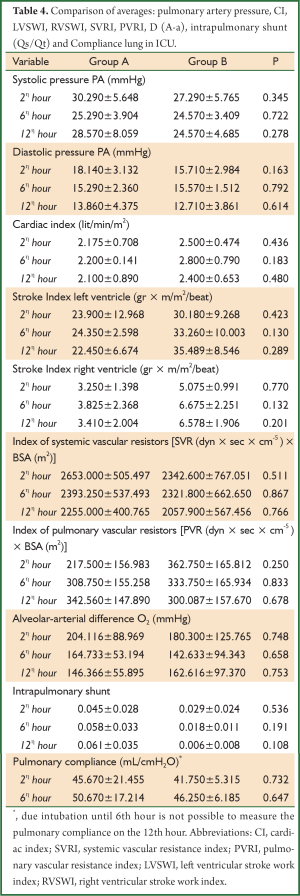
Full table
Indicators of inflammation in the blood
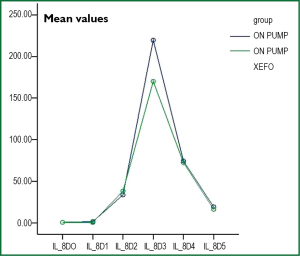
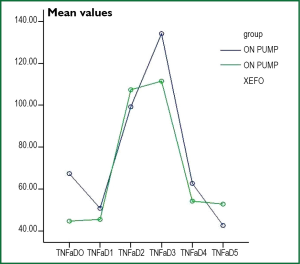
In Table 5 the mean values and the standard deviation of the cytokine (IL-6, IL-8, TNF-a), adhesion molecules (ICAM-1, E-Selectin, P-Selectin) and matrix metalloproteinase-3 (MMP-3) of the patients of both groups are listed. With respect to cytokines, there was no significant difference in IL-8 and TNF-a between the two groups at any sampling period. With respect to IL-6 statistically significant difference between the two patient groups was observed in the sample of the first postoperative days (D5), and in the remaining samples (D0-D4) there was no statistically significant difference. However from the graph of the change in the average value of IL-6 (Figure 2), the IL-8 (Figure 3) and TNF-a (Figure 4) there were observed values in the patient group of lornoxicam lower than those of control patients.
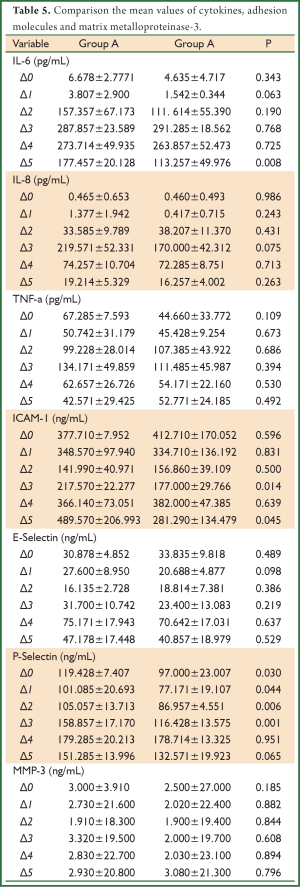
Full table
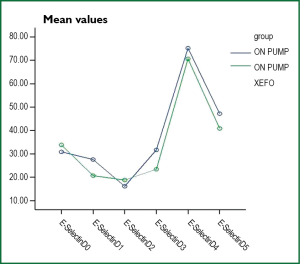
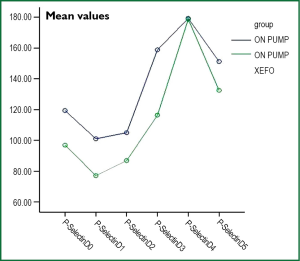
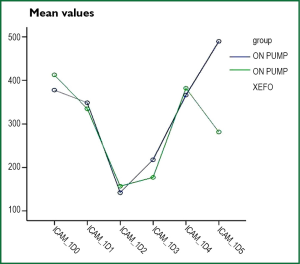
Adhesion molecules exhibit statistically significant differences in some images periods as shown in Table 5. However, the prices of E-Selectin (Figure 5) and P-Selectin (Figure 6) in all samples of patients in the lornoxicam group were lower than the corresponding values of the control patients. The same was observed for the values of ICAM-1 (Figure 7) for both groups of patients. With respect to MMP-3, although in the lornoxicam group was decreased compared to the control group, however, in the comparison of the mean values of all samples of the two groups was not observed any statistically significant difference.
Microscopic study of lung tissue pieces
The results of the study of microscopic pieces of lung tissue showed the following:
Control group (Group A)
The tissues at the end of surgery, as compared to the samples prior to extracorporeal circulation, exhibit adhesion of inflammatory cells of formula eosinophil polymorph nuclear leukocytes to the endothelium of small blood vessels of the lung vasculature, and in the capillaries of the pulmonary alveoli. The meso alveolar septa exhibit low grade oedema. Furthermore in perisplachniou petal pleural except adhesion of eosinophils in the smaller blood vessels it is observed exit of these in the interstitial tissues relaxed. The density of the eosinophil leukocyte averaged at 42±3.5 eosinophil leukocytes per field ×200 magnification. This price is derived from the average of six fields counted (Figure 8).
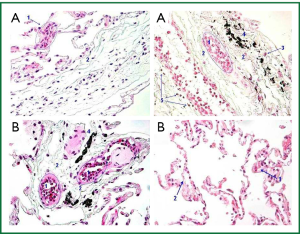
Study group of lornoxicam (Group B)
In four patients the studied tissues at the end of surgery, as compared to those before the extracorporeal circulation, exhibit similar microscopic image. A patient presents in one of the six fields 26 eosinophil polymorph nuclear leucocytes, in magnification ×200. Another patient presents in one of the six fields 34 eosinophil polymorph nuclear leukocytes at ×200 magnification and focal hyperemia of small blood vessels. The last patient of group B presents 39 eosinophil polymorph nuclear leukocytes in all fields, including environ visceral pleura (Figure 8).
Discussion-conclusions
During the extracorporeal circulation, the passage of blood through the tubing system and oxygenator, which are not biological surfaces, results in the activation of a specific (immune) and nonspecific (inflammatory) reaction of various systems. The inflammatory reaction of the body manifests quickly and some patients may officiate in the first minutes, hours or days after using the EC.
The initial reaction of the body is evidently humeral response and begins immediately on contact of the plasma with non-biological surfaces of the pipes of the circuit and the oxygenator. It seems that the oxygenator due to the large gas exchange surface available is the biggest stimulus for the onset of this reaction. The activation of factor Hageman (2) (factor XII may be the trigger of complement activation, kallikrein, the fibrinolytic system, and other procedures, although platelets appear to be activated independently of this mechanism at the same time.
The products of activation of these processes have strong-round effects, direct and indirect, through the activation of other systems and cells. Thus, upon activating the complement cascade (3,4) this leads to the production of potent anaphylatoxins (C3a, C5a), which increase the permeability of blood vessels, causing smooth muscle contraction, stimulating chemo taxis of white blood cells and facilitate the accumulation of neutrophils and the release of enzymes (5,6). On the other hand, the activation of Hageman factor stimulates immediately cataract Kallikrein-bradykinin that has resulted in the production of bradykinin (7). Bradykinin increases the permeability of blood vessels dilate arterioles, causes smooth muscle contraction and pain (8,9). Kallikrein also activates factor XII and activates plasminogen to plasmin, which ultimately lyses fibrin and clots. Thus blood clotting mechanism is disturbed. In the inflammatory reaction of the body the cellular elements of the blood and endothelial cells are involved (10). Significant is the participation of monocytes, once they are activated, in the cellular reaction against inflammation. Also strategic role have neutrophil granulocytes in the reaction of EC when they are activated by complement and other soluble mediators of inflammation. Once these cells are activated (11) they migrate to locations (12) where there is high complement concentration (usually the tissue or during the EC in the blood), they change their shape, becoming more “adherent” (13-17) and secrete cytotoxic substances including free oxygen radicals. It should however be stressed that the supplement may desensitize neutrophils and reduce their ability to participate in the inflammatory reaction. This mechanism explains why the majority of patients after cardiac surgery under EC exhibit uncomplicated recovery. Also neutrophil cell activation it may be made by other factors as kallikrein, the TNF-a, and platelet activating factor (PAF). It has been shown that all these molecules are increased during the EC and immediately after this.
Immediately upon the entry into the EC the activation of platelets begins, the mechanism of which is undetermined, but it is probable that contact with non-biological surfaces and the effect of sheer stress (shear stress) may be the trigger (18). The endothelial cells and other cells, especially those that are local to the ischemic region during the EC (e.g., lung), in the presence of cell membrane phospholipids and products of arachidonic acid (eicosanoids), act as mediators of inflammation with the release of prostaglandins, thromboxanes, leukotrienes and lipoxins.
It becomes obvious that the generalized inflammatory reaction of the body is a complex mechanism, which is released immediately with the start of the EC, and which is responsible for a number of pathological processes in various organs and tissues. Occasionally there have been efforts to reduce the inflammatory reaction, which were turned to search and construction of materials more biocompatible with the human body, while performing coronary surgery with the heart beating was a major effort to minimize this complication (19-25). Another attempt to limit the inflammatory response was the “mini” extracorporeal machine. This is a compact machine, which is placed near the patient’s chest and is characterized by the short length of the circuit piping.
Occasionally they have been used preoperatively and intraoperatively various pharmacological agents (e.g., aprotinin, pentoxifylline, corticosteroids, synthetically produced antibodies against specific mediators of inflammation) in order to reduce or completely eliminate the inflammatory reaction (2,3,5,6,26-37). In particular for the study of inflammatory reaction in the lungs, in the literature they are referred clinical research protocols by using primarily of aprotinin in order to reduce the inflammatory reaction (33,38-40).
In our study the purpose was to determine whether administration of a no steroidal anti-inflammatory agent, lornoxicam, before the start of extracorporeal circulation, could help reduce the inflammatory response in the lungs.
We studied 14 patients, 7 were in the control group (Group A) and 7 of lornoxicam group (Group B) and a total of 35 variables were examined. Although the number of patients is small sample, the results are statistically significant because the two groups included the same number of patients; they were uniformed and showed no statistically significant differences in age, gender, body surface area, predisposing factors (diabetes mellitus, hypertension, smoking), the left ventricular function (ejection fraction), the occurrence of angina, the degree of concomitant heart failure (sorted by NYHA) and elements of control spirometry (FVC, FEV1, FVC/FEV1). Also in both groups of patients, the average number of performed, coronary bypasses, the time of extracorporeal circulation time AOC and the total time of surgery did not show statistically significant difference. Certainly clinical observations could be drawn from a safe only if the sample of the study patients was significantly greater than that was used.
Our study focused on four objectives: (I) in the control of heart function; (II) control of the respiratory function (III) monitoring the indices of inflammatory reaction in the blood and (IV) microscopic examination of tissue particles lung for the study of histological findings.
Regarding the monitoring indicators of cardiac function in both groups of patients, the results of our study showed that the variables of cardiac index, index of systemic and pulmonary vascular resistance, the ratio of work left and right ventricle, did not show any statistically significant difference between the averages of the measurements between the two groups at any of the time points of measurement (P>0.05). Exception was the variable of systolic pulmonary artery pressure in which a single measurement at the end of surgery, was statistically significantly lower in the lornoxicam group compared to the control group (P=0.024), while there was no statistically significant difference in diastolic pulmonary artery pressure. Although the pulmonary artery pressure is a parameter indicative of left ventricular function, however, this finding alone is not a reliable indicator beneficial effect of lornoxicam on cardiac function and therefore it cannot be assessed. Regarding the monitoring indicators of respiratory function (alveolar-arterial oxygen difference, intrapulmonary Shunt) from the study of our results there was at no time measurements statistically significant difference in these parameters (P>0.05) from the study of our results there was at no time measurements with statistically significant difference in these parameters (P>0.05). These findings suggest both the existence of the inflammatory response to low levels (subclinical), but on the other hand there is no response of the lungs to the administration of lornoxicam.
Controversial are the results of the literature in terms of preventing the release of inflammatory reaction in the lungs with the administration of other pharmaceutical agents. Some researchers by providing aprotinin, cortisone or acetylcysteine, managed to reduce lung injury (38,40) of the inflammatory reaction by improving alveolar-arterial oxygen difference and pulmonary compliance, while others failed (39).
The indicators of inflammation in the blood are of great interest. But it must be noted that from the graph of change in the average value of IL-6 (Figure 1), result that lornoxicam brings lower prices than those of the control group.
The IL-6 is increased during and immediately after the EC with greater concentration a few hours after its completion and gradual decline in preoperatively levels in the next 3-5 days. Increase in IL-6 plasma levels is observed after three 2-4 hours from the skin incision. The increase of IL-6 in EC is observed in normothermia, systemic hypothermia, in patients who used heparinized circuits EC (41-43) and in paediatric patients (44), to whom it was found elevated in bronchoalveolar lavage fluid and it was associated with increased postoperative morbidity. Similar results were reported by Liebold A. et al., who found peak of IL-6 six hours after the EC (45,46). With regard to IL-8 and TNF-a it was not recorded statistically significant difference between the averages of the measurements of the two groups in any of the measurement times (P>0.05). From the graph of change in the average value of IL-8 (Figure 2) it was observed that administration of lornoxicam bring in all measurements values that are less than or equal to those of the control group. Also from the graph of the average change in the price of TNF-a (Figure 3) lornoxicam reduces on the expression of this factor.
Different results regarding the timing of increasing IL-8 after the EC (47), who argue that the appearance of blood is made after 24 hours from the EC. The same authors show similar results for factor TNF-a.
Regarding adhesion molecules (icam-1, E-Selectin, P-Selectin) there were fluctuations in our results. The E-Selectin showed no statistically significant difference between the averages of the measurements of the two groups in any of the time points measured (P>0.05), although in the group of pro-LNX it was observed on smaller values of levels of E-Selectin (Figure 4). Similar results are reported in the international literature (48).
Unlike the P-Selectin in the lornoxicam group was statistically significantly lower than the control group in the samples D0-D3 (P<0.05 in all measurements). But at other times of measurements (samples D4, D5) the values of P-Selectin was found lower than those of group A (Figure 5), but on these measurements the difference was not statistically impacted significantly (P=0.951 and P=0.065 respectively).
Wei M. and partners (49) in their study on the fluctuations of adhesion molecules in plasma in patients who had been underwent coronary bypass with or without extracorporeal circulation, indicate that P-Selectin is increased plasma immediately after anaesthesia induction, displays further increase during the EC and about four hours after the EC displays a gradual decrease. Similar were our own results.
Interesting is, the fluctuation of ICAM-1 in both groups of patients. As shown in Figure 6, the release curve of icam-1 patients lornoxicam first displays a fall immediately after the EC and then gradually increases up to the first eight hours after the end of surgery and then it is inverted and shows a gradual decline. In contrast to patients who did not receive lornoxicam curve of ICAM-1 (as shown in Figure 6) continues to rise beyond eight hours, so in the morning of the first postoperative day, the difference in prices between the two groups is statistically significant (P=0.045). The peculiarity of this curve is shown by other authors who argue that this increase continued for 20 hours (49). Similar results were reported by other writers who argue that icam-1 is an indicator presence of respiratory distress, resulting inflammatory response (50).
Finally in values of MMP-3, as an expression marker of inflammation in plasma, there were no statistically significant differences between the average values of the measurements in the two groups in any of the samples (P>0.05). Corresponding measurements with the MMP-3 do not exist in the literature on cardiac surgery. Conversely they have been studied other metalloproteinase such as MMP-8, MMP-13, proMMP-9 and proMMP-2. It has been found that the EC promotes the synthesis and release of metalloproteinase (matrix metalloproteinase) (51). In particular, MMP-8 and MMP-13 are quadrupled at the end of the EC, pro-MMP-9 are tripled after the lifting of the blockade of the aorta and remains with the pro-MMP-2 at high levels for 24 hours after the EC.
Histological examination of lung tissue raises definitively diagnosing of the presence of inflammation in the lung in response to the EC. The finding filtration of the lung with eosinophil granulocytes, the presence of edema in alveolar septa medium and the appearance of local hyperemia are reliable signs suggesting an inflammatory reaction. The degree of occurrence of the above findings determines the intensity of the inflammatory response.
At the end of surgery, the tissues in the control group (Group A), compared to those before extracorporeal circulation, have increased difference in the adhesion of inflammatory cells to the type of eosinophil polymorph nuclear leukocytes. The median alveolar septa exhibit edema, though it is of small degree. It is observed output of eosinophil into perisplachniou petal pleura, the loose interstitial tissues and adhesion of eosinophil in small blood vessels. The density of eosinophil leukocytes on average amounts to 42±3.5 eosinophil leukocytes per field.
In the group of the administration of lornoxicam (Group B) it was observed that in 57.14% of patients (4/7 patients) the microscopic image of the lung after extracorporeal circulation was similar to that before extracorporeal circulation. In 28.57% of patients (2/7 patients), it was observed a slight increase in the number of eosinophil (26 and 34 polymorph nuclear). This increase is designated as restricted as it is illustrated in one of the six fields studied per patient. In 14.29% of patients (1/7 patient) they were counted 39 eosinophil polymorph nuclear leukocytes in all fields, including these of perisplachniou pleura. This increase in the number of eosinophil is important, however, is smaller than that of the control group where the number of the respective cells was 42±3.5 cells per field. However, it should be noted that in any patient of lornoxicam group was observed histologically edema in medium alveolar septa, which is a strong indicator of the inflammatory reaction.
From the results of our study it is demonstrated that the administration of lornoxicam, a no steroidal anti-inflammatory agent, in patients who have been undergone into coronary bypass under extracorporeal circulation:
- It is safe because it does not cause complications of the various organ systems;
- It reduces alveolar-arterial oxygen difference and intrapulmonary shunt, improves lung compliance and consequently the performance of the lungs during the immediate postoperative period;
- It improves cardiac output index, the index of the systemic vascular resistance the index work of the left ventricle and contributes thereby to the better performance of the heart immediately after the procedure;
- It significantly reduces the levels of IL-6, the P-Selectin and ICAM-1 in plasma, which are strong indicators detecting inflammatory reaction after extracorporeal circulation;
- It provides protection against lung inflammatory reaction, since it prevents the creation of edema in medium alveolar septa and interstitial infiltration of lung tissue by inflammatory cells;
- Limitations of our study were: (i) the small number of patients does not allow us to draw conclusions of greater clinical importance and (ii) lack of reference values of indicators of the inflammatory response to large group of healthy people compared to the corresponding values after extracorporeal circulation.
Acknowledgements
Disclosure: the authors declare no conflict of interest.
References
- Pruss TP, Stroissnig H, Radhofer-Welte S, et al. Overview of the pharmacological properties, pharmacokinetics and animal safety assessment of lornoxicam. Postgrad Med J. 1990;66 Suppl 4:S18-21. [PubMed]
- O’Brien JG, Battistini B, Zaharia F, et al. Effects of tranexamic acid and aprotinin, two antifibrinolytic drugs, on PAF-induced plasma extravasation in unanesthetized rats. Inflammation 2000;24:411-29. [PubMed]
- Wachtfogel YT, Harpel PC, Edmunds LH Jr, et al. Formation of C1s-C1-inhibitor, kallikrein-C1-inhibitor, and plasmin-alpha 2-plasmin-inhibitor complexes during cardiopulmonary bypass. Blood 1989;73:468-71. [PubMed]
- Kirklin JK, Chenoweth DE, Naftel DC, et al. Effects of protamine administration after cardiopulmonary bypass on complement, blood elements, and the hemodynamic state. Ann Thorac Surg 1986;41:193-9. [PubMed]
- Sahu A, Lambris JD. Complement inhibitors: a resurgent concept in anti-inflammatory therapeutics. Immunopharmacology 2000;49:133-48. [PubMed]
- Kirklin JK, Westaby S, Blackstone EH, et al. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg 1983;86:845-57. [PubMed]
- Cugno M, Nussberger J, Biglioli P, et al. Cardiopulmonary bypass increases plasma bradykinin concentrations. Immunopharmacology 1999;43:145-7. [PubMed]
- Smith EE, Naftel DC, Blackstone EH, et al. Microvascular permeability after cardiopulmonary bypass. An experimental study. J Thorac Cardiovasc Surg 1987;94:225-33. [PubMed]
- Pacifico AD, Digerness S, Kirklin JW. Acute alterations of body composition after open intracardiac operations. Circulation 1970;41:331-41. [PubMed]
- Vane JR, Anggård EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med 1990;323:27-36. [PubMed]
- Brown GM, Drost E, Selby C, et al. Neutrophil sequestration in rat lungs. Ann N Y Acad Sci 1991;624:316-7. [PubMed]
- Markos J, Hooper RO, Kavanagh-Gray D, et al. Effect of raised alveolar pressure on leukocyte retention in the human lung. J Appl Physiol (1985) 1990;69:214-21. [PubMed]
- Gebb SA, Graham JA, Hanger CC, et al. Sites of leukocyte sequestration in the pulmonary microcirculation. J Appl Physiol (1985) 1995;79:493-7. [PubMed]
- Li X, Abdi K, Rawn J, et al. LFA-1 and L-selectin regulation of recirculating lymphocyte tethering and rolling on lung microvascular endothelium. Am J Respir Cell Mol Biol 1996;14:398-406. [PubMed]
- Donnelly SC, Haslett C. Cellular mechanisms of acute lung injury: implications for future treatment in the adult respiratory distress syndrome. Thorax 1992;47:260-3. [PubMed]
- Ernst E, Hammerschmidt DE, Bagge U, et al. Leukocytes and the risk of ischemic diseases. JAMA 1987;257:2318-24. [PubMed]
- Warshawski FJ, Sibbald WJ, Driedger AA, et al. Abnormal neutrophil-pulmonary interaction in the adult respiratory distress syndrome. Qualitative and quantitative assessment of pulmonary neutrophil kinetics in humans with in vivo 111indium neutrophil scintigraphy. Am Rev Respir Dis 1986;133:797-804. [PubMed]
- Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest 1997;112:676-92. [PubMed]
- Kim HK, Kim WH, Hwang SW, et al. Predictive value of intraoperative transesophageal echocardiography in complete atrioventricular septal defect. Ann Thorac Surg 2005;80:56-9. [PubMed]
- Gormley SM, McBride WT, Armstrong MA, et al. Plasma and urinary cytokine homeostasis and renal function during cardiac surgery without cardiopulmonary bypass. Cytokine 2002;17:61-5. [PubMed]
- Wan S, Izzat MB, Lee TW, et al. Avoiding cardiopulmonary bypass in multivessel CABG reduces cytokine response and myocardial injury. Ann Thorac Surg 1999;68:52-6; discussion 56-7. [PubMed]
- Tárnok A, Hambsch J, Emmrich F, et al. Complement activation, cytokines, and adhesion molecules in children undergoing cardiac surgery with or without cardiopulmonary bypass. Pediatr Cardiol 1999;20:113-25. [PubMed]
- O’Brien PK, Kucharczuk JC, Marshall MB, et al. Comparative study of subxiphoid versus video-thoracoscopic pericardial “window”. Ann Thorac Surg 2005;80:2013-9. [PubMed]
- Ascione R, Lloyd CT, Underwood MJ, et al. On-pump versus off-pump coronary revascularization: evaluation of renal function. Ann Thorac Surg 1999;68:493-8. [PubMed]
- Cleveland JC Jr, Shroyer AL, Chen AY, et al. Off-pump coronary artery bypass grafting decreases risk-adjusted mortality and morbidity. Ann Thorac Surg 2001;72:1282-8; discussion 1288-9. [PubMed]
- Moazami N, Rice TW, Rybicki LA, et al. Stage III non-small cell lung cancer and metachronous brain metastases. J Thorac Cardiovasc Surg 2002;124:113-22. [PubMed]
- Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg 2002;21:232-44. [PubMed]
- Fitch JC, Rollins S, Matis L, et al. Pharmacology and biological efficacy of a recombinant, humanized, single-chain antibody C5 complement inhibitor in patients undergoing coronary artery bypass graft surgery with cardiopulmonary bypass. Circulation 1999;100:2499-506. [PubMed]
- Inaba H, Kochi A, Yorozu S. Suppression by methylprednisolone of augmented plasma endotoxin-like activity and interleukin-6 during cardiopulmonary bypass. Br J Anaesth 1994;72:348-50. [PubMed]
- Wan S, LeClerc JL, Huynh CH, et al. Does steroid pretreatment increase endotoxin release during clinical cardiopulmonary bypass? J Thorac Cardiovasc Surg 1999;117:1004-8. [PubMed]
- Fillinger MP, Rassias AJ, Guyre PM, et al. Glucocorticoid effects on the inflammatory and clinical responses to cardiac surgery. J Cardiothorac Vasc Anesth 2002;16:163-9. [PubMed]
- Segel LD, Follette DM, Castellanos LM, et al. Steroid pretreatment improves graft recovery in a sheep orthotopic heart transplantation model. J Heart Lung Transplant 1997;16:371-80. [PubMed]
- Krakauer T. Pentoxifylline inhibits ICAM-1 expression and chemokine production induced by proinflammatory cytokines in human pulmonary epithelial cells. Immunopharmacology 2000;46:253-61. [PubMed]
- Denizot Y, Karoutsos S, Nathan N. Differential alterations in plasma colony-stimulating factor concentrations after coronary artery bypass graft surgery with extracorporeal circulation. Cytokine 2001;13:314-6. [PubMed]
- Defraigne JO, Pincemail J, Larbuisson R, et al. Cytokine release and neutrophil activation are not prevented by heparin-coated circuits and aprotinin administration. Ann Thorac Surg 2000;69:1084-91. [PubMed]
- Weis M, Pehlivanli S, Meiser BM, et al. Simvastatin treatment is associated with improvement in coronary endothelial function and decreased cytokine activation in patients after heart transplantation. J Am Coll Cardiol 2001;38:814-8. [PubMed]
- Massoudy P, Zahler S, Freyholdt T, et al. Sodium nitroprusside in patients with compromised left ventricular function undergoing coronary bypass: reduction of cardiac proinflammatory substances. J Thorac Cardiovasc Surg 2000;119:566-74. [PubMed]
- Rahman A, Ustünda B, Burma O, et al. Does aprotinin reduce lung reperfusion damage after cardiopulmonary bypass? Eur J Cardiothorac Surg 2000;18:583-8. [PubMed]
- Hill GE, Snider S, Galbraith TA, et al. Glucocorticoid reduction of bronchial epithelial inflammation during cardiopulmonary bypass. Am J Respir Crit Care Med 1995;152:1791-5. [PubMed]
- Tassani P, Richter JA, Barankay A, et al. Does high-dose methylprednisolone in aprotinin-treated patients attenuate the systemic inflammatory response during coronary artery bypass grafting procedures? J Cardiothorac Vasc Anesth 1999;13:165-72. [PubMed]
- Butler J, Chong GL, Baigrie RJ, et al. Cytokine responses to cardiopulmonary bypass with membrane and bubble oxygenation. Ann Thorac Surg 1992;53:833-8. [PubMed]
- Steinberg JB, Kapelanski DP, Olson JD, et al. Cytokine and complement levels in patients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg 1993;106:1008-16. [PubMed]
- Kawamura T, Wakusawa R, Okada K, et al. Elevation of cytokines during open heart surgery with cardiopulmonary bypass: participation of interleukin 8 and 6 in reperfusion injury. Can J Anaesth 1993;40:1016-21. [PubMed]
- Hauser GJ, Ben-Ari J, Colvin MP, et al. Interleukin-6 levels in serum and lung lavage fluid of children undergoing open heart surgery correlate with postoperative morbidity. Intensive Care Med 1998;24:481-6. [PubMed]
- Liebold A, Rödig G, Birnbaum DE. Performance of temporary epicardial stainless steel wire electrodes used to treat atrial fibrillation: a study in patients following open heart surgery. Pacing Clin Electrophysiol 1999;22:315-9. [PubMed]
- Liebold A, Keyl C, Birnbaum DE. The heart produces but the lungs consume proinflammatory cytokines following cardiopulmonary bypass. Eur J Cardiothorac Surg 1999;15:340-5. [PubMed]
- Kalfin RE, Engelman RM, Rousou JA, et al. Induction of interleukin-8 expression during cardiopulmonary bypass. Circulation 1993;88:II401-6. [PubMed]
- Bevilacqua MP, Stengelin S, Gimbrone MA Jr, et al. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science 1989;243:1160-5. [PubMed]
- Wei M, Kuukasjärvi P, Laurikka J, et al. Soluble adhesion molecules and myocardial injury during coronary artery bypass grafting. World J Surg 2003;27:140-4. [PubMed]
- Görlach G, Sroka J, Heidt M, et al. Intracellular adhesion molecule-1 in patients developing pulmonary insufficiency after cardiopulmonary bypass. Thorac Cardiovasc Surg 2003;51:138-41. [PubMed]
- Joffs C, Gunasinghe HR, Multani MM, et al. Cardiopulmonary bypass induces the synthesis and release of matrix metalloproteinases. Ann Thorac Surg 2001;71:1518-23. [PubMed]