The medical and surgical treatment of drug-resistant tuberculosis
Introduction
The burgeoning drug-resistant tuberculosis (DR-TB) epidemic is a public health problem of global importance. Although TB incidence and mortality has decreased in several parts of the world, the overall prevalence of multidrug-resistant tuberculosis (MDR-TB) is increasing in many high-burden countries, particularly in Africa (1). According to the latest WHO statistics, approximately half a million new cases of MDR-TB are diagnosed every year (2). Of these, it is estimated that approximately 40,000 have extensively drug-resistant tuberculosis (XDR-TB). Despite this, limited laboratory capacity and lack of widespread drug susceptibility testing in resource-poor settings means that less than 6% of cases are thought to have been correctly diagnosed (2). In 2011, only one in five of the estimated DR-TB cases among patients notified in the world were enrolled on treatment (3). A large reservoir of patients with undiagnosed DR-TB thus exists that continues to drives person-to-person transmission, and threatens to destabilise global TB control (4,5).
The treatment of patients with DR-TB is complex, and characterised by a longer duration of treatment, the use of less potent but more toxic medications, higher relapse rates, and a lower likelihood of treatment success when compared to drug-susceptible TB (6). Treatment for DR-TB treatment is also considerably more expensive: a recent study by Pooran et al. estimated that despite only comprising 2.2% of the case burden of TB in South Africa, DR-TB consumed 44% of the total national costs of diagnosing and managing all forms of TB (~$158 million) in 2011 (7).
In this review, we outline the diagnosis, medical management and treatment outcomes, and indications and outcomes of adjuvant resectional surgery in the management of DR-TB.
Definitions
MDR-TB is defined as resistance to at least isoniazid and rifampicin, the two most effective first-line antituberculous drugs, while XDR-TB is defined as MDR-TB plus resistance to any fluoroquinolone and any second-line injectable (either kanamycin, amikacin or capreomycin) (3). Pre-XDR-TB refers to MDR-TB resistant to either a second-line injectable drug or a fluoroquinolone.
Other terms such as extremely drug-resistant TB (XXDR-TB) (8) or totally drug-resistant TB (TDR-TB) (9,10) have been used by various authors to described strains with more extensive patterns of resistance (to all first-line and second-line drugs). These reports have given rise to the spectre of so-called “untreatable” TB, which has been sensationalized in the media. However, due to problems with the reliability and reproducibility of in vitro drug susceptibility testing for second-line drugs, no international consensus has been reached about the definition of more extensive resistance patterns, and the term “resistance beyond XDR” is preferred. The relevance of these resistance patterns on outcomes is also an active area of study.
Diagnosis of drug-resistant tuberculosis
Culture-based tests for DR-TB
For many years, the laboratory diagnosis of DR-TB has depended on the demonstration of the presence of M.tb growth in the presence of specific antituberculous drugs—so-called conventional drug-susceptibility testing (DST). Solid agar methods are the diagnostic gold standard (11), while liquid culture methods such as the Bactec MGIT 960 system (Becton-Dickinson, Sparks, MD, USA) have equivalent performance, and are WHO-endorsed (12). However, a lack of laboratory infrastructure in developing countries means that very few countries have access to any DST at all: the WHO reports that globally less than 4% of bacteriologically-positive cases and only 6% of retreatment cases were tested for DR-TB in 2011 (13). Another major disadvantage of these culture-based methods is the long delay (usually several weeks) in obtaining DST results. During these delays, regimens may be used which are not only ineffective, but which encourage the development of further drug resistance, and crucially, allow for resistant disease to be spread. A strategy that aims to control DR-TB must therefore aim not only to increase access to DST, but also to reduce the lead-time for accurate diagnosis (14). New advances in rapid growth- and microscopy-based DST, such as the microscopy observed drug susceptibility (MODS) method and thin layer agar (TLA) technique have shortened the delay to less than two weeks, but are limited by the need for labour-intensive laboratory infrastructure (15). More recently, the direct nitrate reductase assay (NRA), a rapid, low-cost, phenotypic method based on the metabolic activity of M.tb which is usually performed on solid media, has been shown to accurately diagnose DR-TB after ~21 days when performed directly on smear-positive specimens (16).
Molecular DST
New nucleic acid amplification tests (NAATs) promise to reduce the interval between sample acquisition and susceptibility result from weeks to hours, and are also becoming increasingly automated and easy to perform. They have the potential to transform the drug-sensitive and drug resistant TB epidemic in high burden countries by providing rapid DST results at the time of TB diagnosis, increasing the number of cases that are diagnosed with DR-TB and started immediately on the correct treatment, and impacting on transmission rates (17).
Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) is a semi-nested quantitative real-time polymerase chain reaction (PCR) assay that can deliver simultaneous diagnosis of TB and rifampicin resistance in less than two hours. It is an automated, cartridge-based system that can be performed in decentralized locations, outside of reference laboratories and potentially at point-of-care, by staff with minimal laboratory training. It has been widely validated on sputum samples, although reports are also emerging on its accuracy in other respiratory specimens and extrapulmonary samples (18-21). A recent meta-analysis has reported the sensitivity and specificity of the assay for the detection of rifampicin resistance in sputum to be 94.1% and 97.0%, respectively (22). Based on this evidence, the WHO has strongly recommended that Xpert® MTB/RIF, where available, should be the first investigation in all patients suspected of having DR-TB or HIV-associated TB (23). A disadvantage is that Xpert® MTB/RIF does not assay for isoniazid resistance and therefore isoniazid mono-resistance, which has a frequency of about 10-15% in high burden settings, will be missed (24,25). Another concern with Xpert® MTB/RIF is suboptimal positive predictive value in settings where the prevalence of drug-resistant TB is less than 20%. In relatively high-burden settings, even in South Africa where MDR-TB prevalence rates are ~5% to 6% (14), the positive predictive value is only likely to be approximately 70% to 80%, though the precise figure remains unclear. This means that approximately one in three or four rifampicin-resistant results will possibly be falsely positive, creating uncertainty around the decision to start MDR-TB treatment. In South Africa, the policy is to initiate MDR-TB treatment on an Xpert® MTB/RIF showing rifampicin resistance, particularly if the patient is unwell, until further confirmatory test results on two samples (either phenotypic DST or alternative PCR-based test like Hain MTBDRplus) become available.
A line probe assay is laboratory-based type of nucleic acid amplification assay in which products are hybridized onto a nitro-cellulose strip. An example is the MTBDRplus assay (Hain Lifesciences), which offers similar performance to Xpert MTB/RIF for TB detection, and has excellent performance for the detection of MDR-TB (26,27). It has the advantage of interrogating for both rifampicin and isoniazid resistance. More recently, the MTBDRsl assay (second line) assay has been introduced which tests for drug-resistance to second line injectable drugs (mutations on the rrs gene), fluoroquinolones and ethambutol (28). However, this assay has diminished accuracy in smear-negative specimens (29), meaning that culture isolates still need to be awaited to rule-in resistance.
The diagnosis of extrapulmonary DR-TB is even more challenging as obtaining samples for diagnosis often requires specialized skills (e.g., lumbar puncture or biopsy), and the traditional methods of smear microscopy and culture perform poorly on paucibacillary non-sputum samples. Performing an Xpert MTB/RIF on concentrated urine can identify 40% of HIV-TB cases who are sputum-scarce (30), and sensitivity is approximately 23%, 53%, and 78% in the pleural, pericardial and CSF compartments, respectively [R. Meldau and K. Dheda, submitted; S. Pandie and K. Dheda, submitted; (31)]. In the CSF and urine compartments, centrifuging the fluid significantly improved accuracy. In BAL fluid obtained by bronchoscopy, Xpert yield was ~75% and was unaffected by HIV status (18).
Medical management of DR-TB
MDR-TB treatment
The treatment regimen for MDR-TB consists of a backbone of a later generation fluoroquinolone (moxifloxacin, gatifloxacin or levofloxacin) and an injectable aminoglycoside (either amikacin or kanamycin), any first line drug to which the isolate is susceptible, and the addition of group 4 drugs such as cycloserine/terizidone, and ethionamide, such that as least four drugs to which the isolate is likely to be susceptible are being used (see Tables 1,2). The intensive phase (with injectable) is eight months, followed by a continuation phase of 12 to 18 months. The recommended duration of treatment is guided by culture conversion and is usually determined by adding 18 months to the date of the first of consecutive negative cultures; the WHO recommends a total treatment duration of at least 20 months (33). Non-adherence, incorrect drug dosage, hetero-resistance, and malabsorption should be considered in patients who do not show a clinical response and remain persistently culture-positive despite exhibiting consistent susceptibility to second-line drugs. These patients may be considered for the addition of alternative second line agents to their regimens, and/or referred for surgery after appropriate investigations are undertaken.
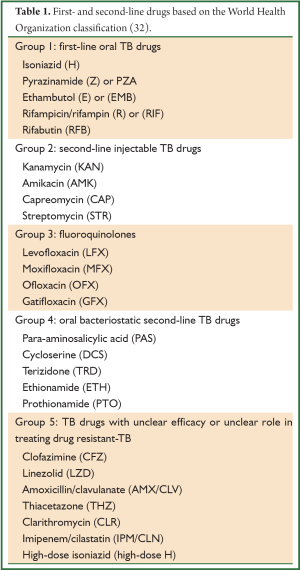
Full table
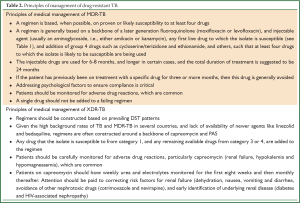
Full table
XDR-TB treatment
With the loss of two of the most potent groups of second-line drugs (namely fluoroquinolones and aminoglycosides), the design of a treatment regimen for XDR-TB is more complex (see Table 2). Extended regimens in TB-endemic countries, given the lack of availability of linezolid, often consist of a backbone of capreomycin and para-aminosalicylic acid (PAS), with other first-line, second-line or third-line anti-TB drugs added to which susceptibility has been demonstrated, or at the discretion of the attending clinician. The intensive phase with capreomycin should be at least eight months (15). The exact number of drugs used to treat XDR-TB is not known, but most patients will receive five to six drugs. Unfortunately, high rates of capreomycin resistance (~80%) in XDR patients have been observed (E. Pietersen and K. Dheda, unpublished work), presumably due to cross-resistance with the other aminoglycosides (34); similarly, as the majority of patients with XDR-TB have been previously treated for MDR-TB (35,36), prior exposure to drugs like ethionamide and terizidone usually excludes their use. Despite documented fluoroquinolone resistance, moxifloxacin is usually added to the regimen because it has increased antituberculous activity compared with ofloxacin, and because there is differential strain-specific susceptibility to the fluoroquinolones (37). Moxifloxacin has been shown to be effective against isolates phenotypically resistant to ofloxacin or ciprofloxacin (38), and may be associated with improved outcomes for patients with XDR-TB (39). In isolates where lack of isoniazid susceptibility results from mutations in the promoter region of the inhA gene (40-42), low-level resistance can likely be overcome by increased doses of the isoniazid (“high-dose INH”) (43). This pattern of resistance is often accompanied with cross-resistance to other second line anti-tuberculosis agents, specifically ethionamide, as it has a structural similarity to isoniazid (44). Other drugs like clofazamine (45) and beta-lactam antibiotics like meropenem and co-amoxiclav (46) from group 5 are also used, although good quality efficacy data is lacking. Bedaquiline, the first novel antituberculous drug to emerge in almost half a century (47), has been cautiously approved in an interim recommendation by the WHO for patients in whom a regimen containing four effective second-line drugs cannot be constructed, or in patients where there is MDR-TB plus documented resistance to a fluoroquinolone (pre-XDR-TB), provided that bedaquiline can be protected by at least three effective drugs (48). The latter is often not possible in TB-endemic countries with currently available drugs. Safety concerns have been raised about the interaction between bedaquiline and clofazamine and the fluoroquinolones, as all cause QT prolongation. The addition of linezolid to the regimen of patients failing standard XDR-TB treatment has been shown to improve culture-conversion, but longer-term outcomes are unknown, and cost and toxicity are major concerns (49). Neither bedaquiline nor linezolid are currently available to National Treatment Programs in countries where XDR-TB is prevalent and endemic.
Monitoring during treatment
Treatment for DR-TB involves the use of toxic medications: drug-associated adverse effects are common, and can frequently interrupt treatment (50). In addition to monitoring sputum cultures, it is essential to monitor renal function and potassium at least monthly during the intensive phase of treatment involving an injectable. Ototoxic hearing loss is common in patients with DR-TB treated with aminoglycosides: a recent study from South Africa found that 57% of patients had developed high-frequency hearing loss after three months of aminoglycoside treatment (51). All patients should therefore be screened monthly with audiometry during the intensive phase of treatment. Thyroid function should be monitored between six and nine months of treatment with ethionamide, prothionamide or PAS, and a full blood count should be checked monthly in patients taking linezolid.
Treatment outcomes in DR-TB
Globally, survival and treatment outcomes of drug-resistant TB vary widely depending on geographical location, regimen choice, duration of treatment, and background prevalence of TB and HIV (6), but in general, correlate with the degree of drug resistance. The overall treatment outcomes are far from satisfactory: the WHO reports that of the estimated half a million MDR-TB patients started globally on treatment in 2009, only 48% were treated successfully (2). Treatment outcomes in XDR-TB are even worse; while the overall success rate for XDR-TB in a recent meta-analysis was reported to be 44% (39), additional resistance to second- and third-line TB medications beyond the minimum definition of XDR-TB was associated with further reductions in the likelihood of success. The cure rate in high-burden countries may be even lower: in South Africa, less than 20% of patients with XDR-TB culture-converted within six months of initiation of treatment, and this poor outcome was independent of HIV status (35). There is thus a desperate need for new drugs and additional interventions to improve these outcomes, particularly in XDR-TB. A number of novel drugs are undergoing clinical testing, but are unlikely to be available for several years yet (52).
Adjuvant surgical management of drug-resistant TB
Rationale and indications for surgery
Thick walled cavitatory lesions and areas of destroyed lung contain up to 107 to 109M.tb organisms (53), harbouring actively replicating bacilli even in patients who are sputum culture-negative (54,55). These tuberculous lesions have reduced exposure to host defenses, and are penetrated poorly by antituberculous drugs (56). Cavities act not only as huge reservoirs of M.tb infection (with the potential for intrapulmonary or contralateral spread), but also as the likely site of the development of drug resistance (57). The rationale behind surgery for DR-TB is that excision of these cavities (along with “debulking” of any necrotic or non-viable lung tissue) will dramatically reduce the overall organism burden in the lung while simultaneously removing the sites of high concentrations of drug resistant bacilli. The surgical removal of cavities is hoped to enhance the sterilizing properties of post-surgical chemotherapy and increasing the likelihood of treatment success (58,59). Complications of TB including massive haemoptysis, aspergilloma, bronchiectasis, pneumothorax, bronchopleural fistula, tracheal or bronchial stenosis and empyema remain valid indications for surgery in both drug-sensitive and drug-resistant TB (60), but these topics are beyond the scope of this review.
The indications for surgery for DR-TB have remained largely unchanged since they were first described by Iseman et al. in 1990 (61), (an adaptation of which is shown in Table 3). Potential surgical candidates include those patients with localized disease and adequate pulmonary reserve who have either: persistently positive sputum smears and/or cultures despite an adequate trial of appropriate chemotherapy; or those who have relapsed, or are thought to be at high risk of relapse based on results of drug resistance profiling or radiological findings. The lack of effective sterilizing chemotherapy for XDR-TB means even “cured” patients remain at high risk for relapse, and may be considered candidates for resection regardless of sputum culture status. The prerequisite of the presence of sufficient susceptible drug activity to facilitate healing of the bronchial stump is also less relevant in the setting of XDR-TB, where extended resistance patterns mean that surgery often remains the only option for cure.

Full table
Timing of surgery
Ideally, surgery should be performed once culture-conversion has been achieved to minimize the risk of post-surgical complications. Performing surgery later in the course of treatment may also allow for time for nutritional supplementation and control of coexisting medical conditions. However, particularly in the case of XDR-TB (as outlined above), this is unlikely to ever be achieved. Delaying surgery and persisting with ineffective chemotherapy may only facilitate progression of disease, and further promote the development of drug resistance (62). The timing of adjuvant surgery must consider the likelihood of potential culture-conversion based on the resistance profile of the M.tb isolate; however, a minimum of three to six months of pre-operative chemotherapy is usually given (62-66).
An additional consideration in the setting of HIV/DR-TB co-infection is that surgery may need to be postponed until immunity has been restored with antiretroviral therapy (ART) to the point at which it is expected that major surgery can be withstood.
Preoperative workup, surgical approach and complications of surgery
The preoperative workup is directed at assessing disease extent and estimating cardiopulmonary reserve (see Table 4). A high-resolution CT chest is a prerequisite to assess the presence of contralateral disease, and to plan the surgical approach. Spirometry is required to estimate pulmonary reserve, while a 6-minute walk test (6MWT) is a good test of functional capacity. In borderline cases, quantitative perfusion lung scanning can assist in estimating the degree of functional loss following surgery. Cut-off values for predicted postoperative lung function are not defined in this group of patients, but can be adapted from studies of resectional surgery for lung cancer: predicted post-operative forced expiratory volume in one second (FEV1) should likely be greater than ~800 mL for pneumonectomy candidates (67). An ECG followed by an echocardiogram, where indicated, may be useful in excluding pulmonary hypertension, which would otherwise contraindicate surgery. Positron emission tomography-computed tomography (PET/CT) has been proposed as a noninvasive imaging method that may give additional information about tuberculous disease status, particularly about the presence of contralateral parenchymal as well as nodal metabolic activity (68,69). Its role in guiding surgery remains unclear. The preoperative improvement of nutritional status has been advocated by many authors (64,65,70,71) to improve wound healing and post-operative recovery.

Full table
The extent of anatomical resection (wedge resection versus segmentectomy, lobectomy or pneumonectomy) is determined by the distribution of radiological disease, and is balanced by the desire to remove as much pathological lung as possible while preserving post-operative pulmonary reserve. While the procedures performed in case series and cohort studies were predominantly lobectomies or pneumonectomies for unilateral disease, sequential resection of bilateral cavities in patients with adequate pulmonary reserve has also been described (70,72,73).
The surgical approach is almost always via a muscle-sparing posterolateral thoracotomy; the median approach has also been studied, but offers limited exposure for left-sided resections (74). Video-assisted thoracoscopic surgery (VATS) is associated with less wound pain, fewer pulmonary complications, and a shorter hospital stay than with a thoracotomy. This technique has recently been shown to be a feasible option for smaller wedge resections and isolated lobectomies in carefully selected patients with less extensive disease (75). The obliteration of the pleural space, the presence of infected lymph nodes in the peribronchial area, and extensive adhesions (between the lung, vasculature and the chest wall) occurring as a result of chronic tuberculous sepsis, typically limit thoracoscopic intervention and are a contraindication to VATS (63).
Bronchial stump closure is usually by stapling, although interrupted sutures with absorbable or non-absorbable sutures (either on their own or as additional reinforcement), are also used (59,65). Muscle-flap buttressing of the bronchial stump has been advocated to prevent the post-operative complication of bronchopleural fistula especially in patients with positive sputum at the time of operation (65); however, this practice has not been universally adopted, and a series of 106 patients from South Africa in which bronchial stump reinforcement was only performed in two cases reported no cases of bronchopleural fistula formation (64).
With careful patient selection, the operative mortality in lung resection is less than 5% (62-66,76). Common complications range in frequency between 12% and 30%, and include bleeding, empyema, wound complications and bronchopleural fistula.
Outcomes of surgical treatment
There is a dearth of good quality of data supporting the use of adjuvant surgical treatment for DR-TB, and current recommendations are based on expert opinion. No randomized controlled trials have been performed, and it is likely that the available data from case series and cohort studies is biased towards surgery in patients with less extensive disease. Nevertheless, a recent systematic review and meta-analysis of 24 comparison studies of MDR- and XDR-TB (involving more than 5,000 patients) found a significant association between surgical intervention and successful outcome when compared to non-surgical treatment alone (OR 2.24, 95% CI: 1.68-2.97) (77). Sub-group analyses of studies involving XDR-TB patients revealed an even more pronounced treatment effect (OR 4.55, 95% CI: 1.32-15.7), which would support the widely held view that surgery as a therapeutic option becomes even more attractive as effective chemotherapeutic options dwindle.
Conclusions
MDR-TB, XDR-TB, and now resistance beyond XDR-TB (TDR or XXDR-TB) are growing epidemics fuelled by failing national TB programs, HIV co-infection, and poverty, and not only have a high mortality but threaten to destabilise many national TB programs. For example, in South Africa, despite drug-resistant TB forming less than 3% of the total case load, it consumes over 40% of the ~US$160 million national TB program budget. There is also the growing problem of therapeutic failures who, because of lack of appropriate alternative facilities, are now being discharged back into the community (78). Although surgery remains a critical part of management, only a small number of patients are amenable to surgery. In TB endemic countries, where it is needed most, qualified thoracic surgeons and surgical facilities are lacking. Even where thoracic facilities are available, there may be hesitation and reluctance amongst some thoracic surgeons to operate on patients with a deadly disease. Even with surgery, outcomes in TB endemic countries are poor. New TB drugs are urgently needed, however, policy makers face an ethical dilemma of making these available as part of drug-sensitive regimens, thus preserving their medium to long term efficacy, or risking shortening the effective lifespan of these drugs by using them first in those with DR-TB. Newer and less costly point-of-care diagnostics for drug-resistant TB are also needed, as is an effective TB vaccine. Above all, however, existing national TB programs need to be strengthened so that drug-resistant TB is prevented. In parallel, the existing case burden needs to be tackled and ongoing transmission minimised.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Streicher EM, Müller B, Chihota V, et al. Emergence and treatment of multidrug resistant (MDR) and extensively drug-resistant (XDR) tuberculosis in South Africa. Infect Genet Evol 2012;12:686-94. [PubMed]
- WHO. Multidrug-resistant tuberculosis (MDR-TB): 2013 Update. Geneva: WHO, 2013.
- WHO. Multidrug and extensively drug-resistant TB (M/XDR-TB). Geneva: WHO, 2009.
- Dheda K, Warren RM, Zumla A, et al. Extensively drug-resistant tuberculosis: epidemiology and management challenges. Infect Dis Clin North Am 2010;24:705-25. [PubMed]
- Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 2010;375:1830-43. [PubMed]
- Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis 2009;9:153-61. [PubMed]
- Pooran A, Pieterson E, Davids M, et al. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PLoS One 2013;8:e54587. [PubMed]
- Migliori GB, De Iaco G, Besozzi G, et al. First tuberculosis cases in Italy resistant to all tested drugs. Euro Surveill 2007;12:E070517.1. [PubMed]
- Udwadia ZF, Pinto LM, Uplekar MW. Tuberculosis management by private practitioners in Mumbai, India: has anything changed in two decades? PLoS One 2010;5:e12023. [PubMed]
- Velayati AA, Masjedi MR, Farnia P, et al. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in iran. Chest 2009;136:420-5. [PubMed]
- Van Deun A, Martin A, Palomino JC. Diagnosis of drug-resistant tuberculosis: reliability and rapidity of detection. Int J Tuberc Lung Dis 2010;14:131-40. [PubMed]
- WHO. The use of liquid TB culture and drug susceptibility testing (DST) in low and medium income settings. Geneva, Switzerland: World Health Organization, 2007.
- World Health Organisation Global Tuberculosis Report 2012 Geneva, Switzerland: World Health Organisation. Available online: http://www.who.int/tb/publications/global_report/en/ (cited 2012 9th November).
- WHO. Towards universal access to diagnosis and treatment of multidrug-resistant and extensively drug-resistant tuberculosis by 2015: WHO progress report 2011. Geneva: WHO, 2011.
- Dheda K, Theron G, Peter JG, et al. TB drug resistance in high-incidence countries. Tuberculosis. 58: European Respiratory Society Journals Ltd, 2012:95-110.
- Martin A, Imperiale B, Ravolonandriana P, et al. Prospective multicentre evaluation of the direct nitrate reductase assay for the rapid detection of extensively drug-resistant tuberculosis. J Antimicrob Chemother 2014;69:441-4. [PubMed]
- Menzies NA, Cohen T, Lin HH, et al. Population health impact and cost-effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: a dynamic simulation and economic evaluation. PLoS Med 2012;9:e1001347. [PubMed]
- Theron G, Peter J, Meldau R, et al. Accuracy and impact of Xpert MTB/RIF for the diagnosis of smear-negative or sputum-scarce tuberculosis using bronchoalveolar lavage fluid. Thorax 2013;68:1043-51. [PubMed]
- Steingart KR. Xpert MTB/RIF test for detection of pulmonary tuberculosis and rifampicin resistance. J Evid Based Med 2013;6:58.
- Peter J, Green C, Hoelscher M, et al. Urine for the diagnosis of tuberculosis: current approaches, clinical applicability, and new developments. Curr Opin Pulm Med 2010;16:262-70. [PubMed]
- Alvarez-Uria G, Azcona JM, Midde M, et al. Rapid Diagnosis of Pulmonary and Extrapulmonary Tuberculosis in HIV-Infected Patients. Comparison of LED Fluorescent Microscopy and the GeneXpert MTB/RIF Assay in a District Hospital in India. Tuberc Res Treat 2012;2012:932862.
- Chang K, Lu W, Wang J, et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect 2012;64:580-8. [PubMed]
- WHO. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Geneva: World Health Organization, 2011.
- Rishi S, Sinha P, Malhotra B, et al. A comparative study for the detection of Mycobacteria by BACTEC MGIT 960, Lowenstein Jensen media and direct AFB smear examination. Indian J Med Microbiol 2007;25:383-6. [PubMed]
- Smith SE, Kurbatova EV, Cavanaugh JS, et al. Global isoniazid resistance patterns in rifampin-resistant and rifampin-susceptible tuberculosis. Int J Tuberc Lung Dis 2012;16:203-5. [PubMed]
- Barnard M, Albert H, Coetzee G, et al. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J Respir Crit Care Med 2008;177:787-92. [PubMed]
- Barnard M, Gey van Pittius NC, van Helden PD, et al. The diagnostic performance of the GenoType MTBDRplus version 2 line probe assay is equivalent to that of the Xpert MTB/RIF assay. J Clin Microbiol 2012;50:3712-6. [PubMed]
- Rutledge JA, Crouch JB. The ultimate results in 1654 cases of tuberculosis treated at the Modern Woodmen of America Sanitorium. Am Rev Tuberc 1919;2:755-63.
- Hillemann D, Rüsch-Gerdes S, Richter E. Feasibility of the GenoType MTBDRsl assay for fluoroquinolone, amikacin-capreomycin, and ethambutol resistance testing of Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol 2009;47:1767-72. [PubMed]
- Peter JG, Theron G, Muchinga TE, et al. The diagnostic accuracy of urine-based Xpert MTB/RIF in HIV-infected hospitalized patients who are smear-negative or sputum scarce. PLoS One 2012;7:e39966. [PubMed]
- Patel VB, Theron G, Lenders L, et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: a prospective study. PLoS Med 2013;10:e1001536. [PubMed]
- WHO. Treatment of Tuberculosis: Guidelines. 4th ed. Geneva: WHO, 2009.
- WHO. Guidelines for the progammatic management of drug-resistant tuberculosis: 2011 update. Geneva: WHO, 2011.
- Almeida Da Silva PE, Palomino JC. Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: classical and new drugs. J Antimicrob Chemother 2011;66:1417-30. [PubMed]
- Dheda K, Shean K, Zumla A, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet 2010;375:1798-807. [PubMed]
- Sotgiu G, Ferrara G, Matteelli A, et al. Epidemiology and clinical management of XDR-TB: a systematic review by TBNET. Eur Respir J 2009;33:871-81. [PubMed]
- Cox H, Ford N, Keshavjee S, et al. Rational use of moxifloxacin for tuberculosis treatment. Lancet Infect Dis 2011;11:259-60. [PubMed]
- Kam KM, Yip CW, Cheung TL, et al. Stepwise decrease in moxifloxacin susceptibility amongst clinical isolates of multidrug-resistant Mycobacterium tuberculosis: correlation with ofloxacin susceptibility. Microb Drug Resist 2006;12:7-11. [PubMed]
- Jacobson KR, Tierney DB, Jeon CY, et al. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 2010;51:6-14. [PubMed]
- Sirgel FA, Donald PR, Odhiambo J, et al. A multicentre study of the early bactericidal activity of anti-tuberculosis drugs. J Antimicrob Chemother 2000;45:859-70. [PubMed]
- Jayaram R, Shandil RK, Gaonkar S, et al. Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 2004;48:2951-7. [PubMed]
- de Steenwinkel JE, de Knegt GJ, ten Kate MT, et al. Time-kill kinetics of anti-tuberculosis drugs, and emergence of resistance, in relation to metabolic activity of Mycobacterium tuberculosis. J Antimicrob Chemother 2010;65:2582-9. [PubMed]
- Katiyar SK, Bihari S, Prakash S, et al. A randomised controlled trial of high-dose isoniazid adjuvant therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2008;12:139-45. [PubMed]
- Rist N. The antitubercular activity of ethioniamide (alpha-ethylthioisonicotinamide or Th 1314). An experimental and clinical study. Bibl Tuberc 1960;15:69-126. [PubMed]
- Gopal M, Padayatchi N, Metcalfe JZ, et al. Systematic review of clofazimine for the treatment of drug-resistant tuberculosis. Int J Tuberc Lung Dis 2013;17:1001-7. [PubMed]
- Gonzalo X, Drobniewski F. Is there a place for β-lactams in the treatment of multidrug-resistant/extensively drug-resistant tuberculosis? Synergy between meropenem and amoxicillin/clavulanate. J Antimicrob Chemother 2013;68:366-9. [PubMed]
- Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med 2009;360:2397-405. [PubMed]
- WHO. The use of bedaquiline in the treatment of multidrug-resistant tuberculosis: interim policy guidance. Geneva: WHO, 2013.
- Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 2012;367:1508-18. [PubMed]
- Shean K, Streicher E, Pieterson E, et al. Drug-associated adverse events and their relationship with outcomes in patients receiving treatment for extensively drug-resistant tuberculosis in South Africa. PLoS One 2013;8:e63057. [PubMed]
- Harris T, Bardien S, Schaaf HS, et al. Aminoglycoside-induced hearing loss in HIV-positive and HIV-negative multidrug-resistant tuberculosis patients. S Afr Med J 2012;102:363-6. [PubMed]
- Field SK, Fisher D, Jarand JM, et al. New treatment options for multidrug-resistant tuberculosis. Ther Adv Respir Dis 2012;6:255-68. [PubMed]
- Canetti G. Present aspects of bacterial resistance in tuberculosis. Am Rev Respir Dis 1965;92:687-703. [PubMed]
- Dravniece G, Cain KP, Holtz TH, et al. Adjunctive resectional lung surgery for extensively drug-resistant tuberculosis. Eur Respir J 2009;34:180-3. [PubMed]
- Park SK, Kim JH, Kang H, et al. Pulmonary resection combined with isoniazid- and rifampin-based drug therapy for patients with multidrug-resistant and extensively drug-resistant tuberculosis. Int J Infect Dis 2009;13:170-5. [PubMed]
- Kempker RR, Rabin AS, Nikolaishvili K, et al. Additional drug resistance in Mycobacterium tuberculosis isolates from resected cavities among patients with multidrug-resistant or extensively drug-resistant pulmonary tuberculosis. Clin Infect Dis 2012;54:e51-4. [PubMed]
- Kaplan G, Post FA, Moreira AL, et al. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun 2003;71:7099-108. [PubMed]
- Kempker RR, Vashakidze S, Solomonia N, et al. Surgical treatment of drug-resistant tuberculosis. Lancet Infect Dis 2012;12:157-66. [PubMed]
- Lalloo UG, Naidoo R, Ambaram A. Recent advances in the medical and surgical treatment of multi-drug resistant tuberculosis. Curr Opin Pulm Med 2006;12:179-85. [PubMed]
- Gimferrer JM, Mestres CA. Role of surgery in drug-resistant pulmonary tuberculosis. Asian Cardiovasc Thorac Ann 2005;13:201-2. [PubMed]
- Iseman MD, Madsen L, Goble M, et al. Surgical intervention in the treatment of pulmonary disease caused by drug-resistant Mycobacterium tuberculosis. Am Rev Respir Dis 1990;141:623-5. [PubMed]
- Man MA, Nicolau D. Surgical treatment to increase the success rate of multidrug-resistant tuberculosis. Eur J Cardiothorac Surg 2012;42:e9-12. [PubMed]
- Kang MW, Kim HK, Choi YS, et al. Surgical treatment for multidrug-resistant and extensive drug-resistant tuberculosis. Ann Thorac Surg 2010;89:1597-602. [PubMed]
- Naidoo R. Active pulmonary tuberculosis: experience with resection in 106 cases. Asian Cardiovasc Thorac Ann 2007;15:134-8. [PubMed]
- Pomerantz BJ, Cleveland JC Jr, Olson HK, et al. Pulmonary resection for multi-drug resistant tuberculosis. J Thorac Cardiovasc Surg 2001;121:448-53. [PubMed]
- Pomerantz M, Madsen L, Goble M, et al. Surgical management of resistant mycobacterial tuberculosis and other mycobacterial pulmonary infections. Ann Thorac Surg 1991;52:1108-11; discussion 1112. [PubMed]
- Miller JI Jr. Physiologic evaluation of pulmonary function in the candidate for lung resection. J Thorac Cardiovasc Surg 1993;105:347-51; discussion 351-2. [PubMed]
- Kosterink JG. Positron emission tomography in the diagnosis and treatment management of tuberculosis. Curr Pharm Des 2011;17:2875-80. [PubMed]
- Soussan M, Brillet PY, Mekinian A, et al. Patterns of pulmonary tuberculosis on FDG-PET/CT. Eur J Radiol 2012;81:2872-6. [PubMed]
- Kir A, Inci I, Torun T, et al. Adjuvant resectional surgery improves cure rates in multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg 2006;131:693-6. [PubMed]
- Gegia M, Kalandadze I, Kempker RR, et al. Adjunctive surgery improves treatment outcomes among patients with multidrug-resistant and extensively drug-resistant tuberculosis. Int J Infect Dis 2012;16:e391-6. [PubMed]
- Shiraishi Y, Nakajima Y, Katsuragi N, et al. Resectional surgery combined with chemotherapy remains the treatment of choice for multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg 2004;128:523-8. [PubMed]
- Park SK, Lee CM, Heu JP, et al. A retrospective study for the outcome of pulmonary resection in 49 patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2002;6:143-9. [PubMed]
- Connery CP, Knoetgen J 3rd, Anagnostopoulos CE, et al. Median sternotomy for pneumonectomy in patients with pulmonary complications of tuberculosis. Ann Thorac Surg 2003;75:1613-7. [PubMed]
- Yen YT, Wu MH, Lai WW, et al. The role of video-assisted thoracoscopic surgery in therapeutic lung resection for pulmonary tuberculosis. Ann Thorac Surg 2013;95:257-63. [PubMed]
- Iddriss A, Padayatchi N, Reddy D, et al. Pulmonary resection for extensively drug resistant tuberculosis in Kwazulu-Natal, South Africa. Ann Thorac Surg 2012;94:381-6. [PubMed]
- Marrone MT, Venkataramanan V, Goodman M, et al. Surgical interventions for drug-resistant tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis 2013;17:6-16. [PubMed]
- Dheda K, Migliori GB. The global rise of extensively drug-resistant tuberculosis: is the time to bring back sanatoria now overdue? Lancet 2012;379:773-5. [PubMed]


