Vaccine development for human mastadenovirus
Introduction
Human mastadenovirus (HAdVs) play an important role in a broad spectrum of illnesses in both pediatric and adult patients, including acute respiratory diseases (ARD), acute gastroenteritis and epidemic keratoconjunctivitis (1-3). To date, seven species (A–G) including more than 79 genotypes have been characterized and defined using a new paradigm based on genomics (Table 1) (4). Specific species genotypes are often associated with particular clinical manifestations (5-7). HAdV species B, which contains several important types associated with ARD, can be further divided into two subspecies B1 and B2 according to their tissue tropism. HAdV types 3 and 7 of species B are most commonly detected in patients with respiratory infections (8,9). Since 2006, two more virulent strains HAdV14p1 and HAdV55 of species B have re-emerged resulting in outbreaks and epidemics of severe respiratory disease worldwide (10-17). These two strains have the potential to spread widely and cause severe epidemics due to the lack of herd immunity and their ability to cause more severe ARD than other adenoviruses (18). The only approved live oral vaccine comprising HAdV types 4 and 7 has been used in the US military for 40 years, but has not been approved for use in the general population (19,20). Therefore, there is currently no vaccine approved for general use in children and adults and there is currently no efficient antiviral therapy against adenoviruses. This highlights the need for licensed vaccine development to prevent adenovirus infection outbreaks.

Full table
In this review article, we will summarize the available data on adenovirus epidemiology and the history of the adenovirus vaccine approved for use in the US military. In addition, we will provide a brief overview of innovative vaccine strategies and current progress in the development of animal models for adenovirus vaccine evaluation.
Epidemiology
Adenoviruses commonly cause respiratory diseases, including the common cold, tonsillitis, bronchitis and pneumonia, but also cause other diseases, such as gastroenteritis, cystitis, conjunctivitis, carditis, and rare neurological disorders. Adenovirus infections can occur in patients of all ages and susceptible populations include children, military recruits and immunocompromised patients (1-3).
Infection of children
Endemic, epidemic and sporadic adenovirus infections occur throughout the world. Adenovirus species B, C and E can cause respiratory tract infections accounting for 3–5% of cases of clinically acute respiratory infection. HAdV-1, -2 and -5 of species C usually cause local sporadic infections, whereas HAdV-3, -7, -14 and -21 of species B and HAdV-E4 often cause epidemic outbreaks. HAdV types of species C commonly infect the upper respiratory tract, and HAdV-3, -4 and -7 and the recently reemerged types 14 and 55 often lead to severe respiratory tract disease (21). The incidence rate of human adenovirus pneumonia is only lower than that of respiratory syncytial virus pneumonia in children with viral pneumonia. Adenovirus pneumonia is usually more serious, leading to heart failure, respiratory failure, and encephalitis, myocarditis, liver damage with poor prognosis. In the late 50s to early 60s of last century large-scale adenovirus pneumonia epidemic occurred in children at age of 6 months to 3 years old in northern China with mortality rate as high as 30%, and the main pathogens were confirmed to be HAdV type 3 and 7 (22).
In China, adenovirus infections in children show clear seasonal patterns according to geographical area and the weather conditions. The adenovirus infection rates in northern China are relatively high in autumn and winter, and infection rates peak in southern China in spring and summer (23). Different rates of prevalence of adenovirus types have been noted among children between north and south China, with HAdV-7 being predominant in the north compared with HAdV-3 in the south. HAdV-7 is an important pathogen associated with severe pneumonia in children, and the predominant genotype has shifted from HAdV-7b to HAdV-7d in China as a consequence of continuous evolution. Genome sequencing, phylogenetic analysis and restriction endonuclease analysis comparisons with previous genotypes indicated that HAdV-7d has re-emerged as a major epidemic genotype in southern China (24). In Taiwan, adenovirus type 7 infection is more likely to cause severe pneumonia and death in children than type 3 (25). In addition, HAdV-14p1 and -55 were recently reported in children (26,27), and HAdV-55 has become a common pathogen causing pneumonia among children in northern China.
Acute diarrhea is one of the most common diseases in infants and young children worldwide. Most adenovirus-associated diarrhea studies in children have focused on community-acquired diarrhea and HAdV species F, which is termed enteric adenovirus consisted of HAdV-40 and -41 (28). Enteric adenovirus is an important pathogen in children with diarrhea, second only to rotavirus. Enteric adenovirus infections generally occur in young children under 2 years of age and are more frequent among the summer months. In recent years, the HAdV-40 infection rate has gradually declined; by contrast, the HAdV-41 infection rate has gradually increased (29).
Infection of military recruits
Adenovirus infections are common among military recruits likely due to stressful conditions and high-density living. A large number of researchers have shown that adenoviruses are the major pathogens associated with febrile ARD outbreaks in the US military, and the most commonly detected types are HAdV-4, -7, -14 and -21. In 2006–2007, a relatively rare adenovirus serotype 14 strain was found to be responsible for a number of cases of severe lower respiratory tract disease, resulting in at least 10 deaths and 140 cases of respiratory illness in New York, Oregon, Washington and Texas, among both civilian and military populations. Outbreaks of HAdV-14 infection were then also reported in Europe and China (14-17,30-32). The re-emerging HAdV-14 belonged to a new genome type designated “HAdV-14p1” (also known as “14a”), and the most notable genetic difference between the variant and this prototype HAdV-14 strain was a deletion of 6 base pairs in the fiber knob gene (15).
The main adenovirus types associated with ARD reported in recent years among China’s military were HAdV7 and HAdV-55. The number of outbreaks of HAdV-7-associated ARD that have affected military recruits in China have increased in recent years, with outbreaks consecutively occurring in Shaanxi, Hangzhou, Gansu (33,34). HAdV55 was first identified as HAdV-B11a, which was isolated from a military trainee during an outbreak in Spain in 1969. HAdV-B55 re-emerged in 2005 in Singapore (35,36). In 2006, HAdV-55 QS strain was detected in an outbreak of ARD in Shaanxi province, China. Since then, HAdV-B55 has led to many outbreaks in China among military recruits and civilians, and has become one of the main pathogens causing pneumonia. Strain HAdV-B55 is associated with more severe pneumonia than other strains of this adenovirus. Bioinformatic analysis demonstrated that HAdV55 evolved from an intertype recombination event in the hexon gene between HAdV-B11, a renal pathogen, and HAdV-B14, a respiratory pathogen, of the same subspecies B2 (35-39).
Infection of immunocompromised patients
Adenoviruses play a particularly important role in immunocompromised patients including patients who have received organ or bone marrow transplants and malnourished children, in whom viral diseases are associated with high morbidity and mortality. In immunocompromised patients, adenovirus infections can persist and cause other complications or even death. The most commonly reported adenovirus types include HAdV-C1, -C2, -C5, -A12, -A31, -B3, -B11, -B16, -B34 and -B35, with a predominance of species C (40). In patients who have received bone marrow transplants, adenovirus infections, mainly of types B11, B34 and B35, are responsible for half of all fatal cases, with clinical manifestations including fever, severe pneumonia, liver failure, kidney inflammation or colitis (41).
The history of adenovirus vaccine use in the US military
In the 1940s, adenovirus infections, particularly with HAdV-4 and -7, affected 80% of military trainees and caused epidemic respiratory disease outbreaks among training camps in the US. During the winter months, up to 90% of all hospital admissions for acute respiratory illness in military recruitment camps are caused by adenovirus. This results in significant losses in military manpower, high medical costs and serious disruption to troop training programs. Consequently, there is interest in the development of effective adenovirus vaccines (42).
From 1963 to 1971, the American Institute successfully developed and tested a live, oral, enteric-coated adenovirus vaccine comprising types 4 and 7, and found it to be safe and effective at protecting military trainees from ARD (43,44). In 1980, the FDA approved this adenovirus vaccine for use on military personnel, reducing ARD rates among military trainees to low levels. However, in 1994, the manufacturer of this adenovirus vaccine stopped production due to a lack of financial support to update its vaccine manufacturing facility. By 1999, supplies were exhausted and adenovirus-associated disease, especially serotype 4-associated febrile respiratory illness, returned to military basic training installations (45).
A study by Gray and coworkers predicted that loss of the adenovirus vaccine would be responsible for 10,650 preventable infections, 4,260 medical clinic evaluations, and 852 hospitalizations among the estimated 213,000 active-duty and reserve army, navy, and Marine Corps trainees enrolled each year (46,47). Advisory bodies persuaded Department of Defense leaders to initiate restoration of the adenovirus vaccine. In 2011, after 10 years of investment by government and contractor personnel at a cost of about $100 million, the adenovirus vaccine was restored for use at all military basic training installations. The new vaccine was manufactured to be administered as two separate tablets, one tablet containing adenovirus type 4 and one tablet containing adenovirus type 7. The strains of adenovirus to be used as seeds for production of the live virus active pharmaceutical ingredient were derived from stocks used by the former manufacturer. All other materials were as close to those previously used as possible. In 2011, the FDA licensed marketing of live, oral adenovirus type 4 and 7 vaccines. Disease caused by adenovirus and adenovirus serotype 4 isolation rates have fallen dramatically since vaccinations resumed in October 2011 and remain low. After vaccination was resumed, adenovirus type 14 became the predominant type detected among the US military (48,49). Mindful of the adage that “The more successful a vaccine is, the more quickly the need for it will be forgotten,” sustainment of the supply of the adenovirus vaccine may be a challenge, and careful management will be crucial (43).
Adenovirus vaccine on development
The live oral adenovirus vaccine has been shown to be safe and highly effective in numerous clinical trials and by ongoing surveillance of febrile respiratory illness among US military personnel. However, the live oral enteric-coated adenovirus type 4 and 7 vaccine is approved only for use in military populations of 17–50 years of age. It is advised that the tablets be swallowed whole, without chewing, to avoid release of the virus into the upper respiratory tract. Adenovirus is extremely stable and resistant in natural environments and it is possible that the environment could become polluted by the excreta of subjects. Therefore, for obvious reasons, a vaccine containing a live wild strain of adenovirus should not be recommended for use in children or the general population. Furthermore, the viral strains used in the current vaccine are adenovirus types 4 and 7 that were isolated in the 1960s. Although the adenovirus genome is thought to display long-term stability and the effectiveness of continued adenovirus vaccination with old strains among the US military has demonstrated this, random mutations and homologous recombination events could occur in adenovirus that would result in changes to its antigenicity. In addition, the epidemic types of adenovirus have changed over time and the highly pathogenic HAdV-14 and -55 have re-emerged as prevalent strains worldwide in recent years. Finally, the prevalence of adenovirus types may differ geographically, for example, in China, the most prevalent types associated with ARD are HAdV types 3, 7 and 55. Therefore, it is necessary to develop a novel adenovirus vaccine reflecting currently circulating adenovirus strains (Table 2).
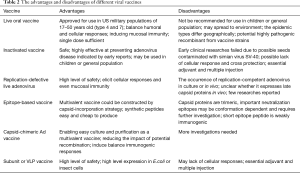
Full table
Inactivated vaccine
Several studies conducted between 1956 and 1960 demonstrated that inactivated adenovirus vaccines were highly effective at preventing adenovirus disease among military recruits (42-45). The bivalent vaccine contained type 4 and 7 adenoviruses, which were cultured in monkey kidney tissue and then formalin-inactivated. However, in later studies, variations in the antigenic potency of different lots of vaccine and failure to obtain consistently high protection rates were noted (50). Moreover, it was found that inactivated vaccines may be contaminated with oncogenic simian virus SV-40. Although a later study found that the tumorigenic properties were highly susceptible to formaldehyde (51,52), the inadequacy of inactivated vaccines urged the development of a live, oral, enteric-coated adenovirus vaccine. Over several decades of use, no evidence has been reported to suggest the oncogenic capacity of the live vaccine strains (20). However, inactivated adenovirus vaccines should be reconsidered due to their higher safety in general population and children, which could be cultured in human diploid cells such as WI-38 or MRC-5 cell line instead of monkey kidney tissue. We found adenovirus could be completely inactivated by β-propiolactone (BPL; final concentration of 0.05%) for 24 h, and the inactivated adenovirus virions elited strong immunogenic responses in mice (unpublished data).
Vaccine epitopes
It is important to identify neutralizing epitopes when designing novel vaccines, antiviral drugs or rapid diagnostic reagents. The adenovirus capsid icosahedron is composed of three major structural proteins: hexon, penton base, and fiber. Based on studies of HAdV-5, the dominant neutralizing antibodies (NAbs) are directed primarily against the hexon hypervariable regions (HVRs), whereas subdominant but still functionally relevant NAbs are directed against the fiber knob in sera from vaccinated mice, vaccinated humans and naturally-exposed humans (53-55). Our research into HAdV-3 packaged by the HAdV-7 hexon confirmed these findings (56). The hexon consists of a pentahedron base and triangles in the tower area comprising four loops (Loop1–4). Loop1 and 2 are composed of seven HVRs, within which type-specific neutralizing epitopes of the hexon have been proposed to reside by multiple sequence alignment and homology modeling methods (57-59) (Figure 1A,B). The fiber protein is composed of three portions, tail, shaft and knob, containing group-specific as well as type-specific epitopes (Figure 1C). The recombinant fiber knob can specifically distinguish antibodies against different HAdV types, indicating that the trimeric knob would be a good candidate antigen for detecting HAdV serotype-specific NAbs in sera from naturally-infected subjects (60,61).
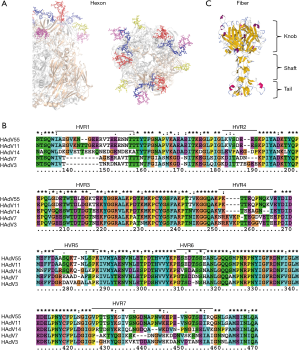
Elucidation of the 3D conformation of the hexon protein would be helpful in determining accurate epitopes; however, X-ray crystal diffraction has only been successfully performed on a few hexon structures. Research groups have previously predicted the B cell epitopes using homology modeling methods to determine the 3D structure of the adenovirus hexon. These putative epitopes have then been verified by ELISA in combination with neutralization tests, indicating the reliability of this epitope mapping method (57-59). Some neutralizing epitopes of HAdV-3, -14 and -55 have been identified with synthetic peptides by this method. Synthetic peptides may be used as vaccine candidates (57,62,63). However, the hexon protein is trimeric and many epitopes may be conformation dependent. Our research confirmed that the major neutralization epitope within HVR5 of HAdV-7 is conformation dependent with four neutralizing monoclonal antibodies and synthetic peptides. This feature increases the difficulty of predicting and identifying neutralizing antigen epitopes (64). The neutralizing mechanism of NAbs against the hexon protein remains unclear and requires further investigation.
Antigen capsid-incorporation is becoming a promising strategy for vaccine development against infectious agents. Previous studies have verified that exogenous antigenic peptides can be incorporated into the hexon HVRs without compromising virus viability, and can effectively trigger a robust antigen-specific immune response. Our group has successfully constructed a recombinant HAdV-3 vector designated Ad3EGFP as a tool for viral delivery or live-vaccine construction. By replacing the predicted epitopes within the HAdV-3 hexon with the corresponding epitopes from the HAdV-7 hexon, the serotype-specific NAb epitopes of HAdV-3 and -7 were identified (59). The important adenovirus types causing respiratory tract infections include HAdV-3, -4, -7, -14 and -55, all of which can result in serious infections such as severe pneumonia and even death in young children and adults. Therefore, it is necessary to develop a new type of adenovirus vaccine, ideally a multivalent vaccine. Through this capsid-incorporation strategy we have obtained several bivalent vaccine candidates against HAdV-3/-7, HAdV-3/-14 or HAdV-3/-55 (59,62,63). This method could also be used to construct a recombinant multivalent adenovirus vaccine.
Genetically engineered vaccine
Replication-defective adenovirus vectors have been successfully used for vaccination and gene therapy against cancer and infectious diseases. Such viruses are unable to replicate in normal cells due to a deletion in the E1 region, which is also responsible for oncogenicity (Figure 2). The replication-defective adenovirus types 3 and 7, among others, may be feasible vaccine candidates due to their high level of safety. However, the occurrence of replication-competent adenovirus (RCA) is a consideration with this type of vaccine. RCA could be greatly minimized using next-generation complementing cell lines. Replication-deficient HAdV-5 can grow in complementary cells integrated with the HAdV-5 E1 gene region, such as the well-known HEK293 cell line. However, RCA may occur via integration of the E1 gene into the recombinant virus genome by homologous DNA recombination. To minimize the occurrence of RCA, next-generation E1 complementing cell lines were developed by minimizing the integrated E1 gene fragment, such as PER.C6, N52.E6 and GH329. However, other species of recombinant adenoviruses in which the E1 gene is deleted cannot replicate in HEK293. By expressing the E1B-55K protein in HEK293 cells, other species of replication-deficient adenoviruses such as HAdV-7 can replicate in these cells (Figure 2B). This interspecies specificity also involves the E4-ORF6 gene (65). This finding makes it possible to prepare other species of replication-defective adenoviruses in HEK293 cells by replacing their E4-ORF6 gene with that of Ad5 (Figure 2C).
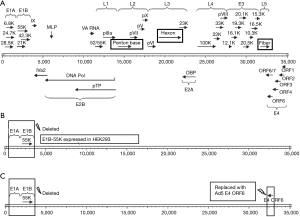
Live replication-defective adenovirus has safe advantage and possible advantage of inducing cellular immunologic response. However, because it may not express late capsid proteins in vivo due to the deletion of early gene, replication-defective adenovirus may require a larger dose than live RCA vaccine. Up to now, few researches are reported using replication-defective adenovirus as adenovirus vaccine. This kind vaccine should be further developed and assessed. However, the general system for the generation of novel recombinant adenoviral vectors is either time-consuming or expensive. So, some new cloning approaches could be introduced for simple and rapid generation of adenovirus vector, such as SLiCE cloning, homologous recombination enzyme exnase cloning, and lethal CcdB screening.
Professor Zhang of Southern Medical University used the replication-defective adenovirus type 5 vector expressing the full-length HAdV-3 hexon as a vaccine candidate against HAdV-3. Our team replaced the major neutralizing antigen hexon of HAdV-3 with the HAdV-7 or -14 hexon to generate a vaccine against HAdV-7 or -14 (56,66). These recombinant viruses have the same genome sequence as HAdV-3 with the exception of the hexon gene, thereby reducing the impact of recombination between different viral strains and enabling easy culturing and purification as a multivalent vaccine.
Some researchers demonstrated that recombinant fowl adenovirus proteins might also be used as subunit vaccine candidates, including recombinant fiber, fiber knob and purified trimeric hexon (67). These results indicate that fiber, fiber knob, virus-like particles (VLP) or trimeric hexon, which would be expressed in E.coli or insect cells, may be developed as human adenovirus vaccine candidates.
Animal models for adenovirus vaccine evaluation
Animal models that support adenovirus infection, replication and pathogenesis are critical for the study of these viruses and the development of prophylactic vaccines and therapeutic medicines. Animal models of adenovirus infection established to date include cotton rat, hamster, New Zealand rabbit, pig, and primate (68-72). However, most of these have been established for HAdV-5 and few animal models have been reported for other adenovirus types (Table 3). Conventional experimental animals as human adenovirus infection models are restricted by two factors: virus receptors and host range restriction factor (HRRF).

Full table
Virus receptors
The receptors of some human adenoviruses have not yet been identified. Unlike most adenovirus serotypes of species A, C, D, E, F and G that use coxsackie and adenovirus receptor (CAR) as their primary attachment receptor, species B serotypes infect cells through the receptor desmoglein 2 (DSG2) or CD46. Adenoviruses that use CAR as their receptor can infect rodent cells because of the high homology of this receptor between humans and rodents, but cannot replicate in these cells due to HRRF. Wang and colleagues suggested a new grouping of HAdV-B based on receptor usage. Group 1 (HAdV-16, -21, -35, -50) almost exclusively use CD46 as a receptor; Group 2 (HAdV-3, -7, -14, -55) share a common DSG2 receptor; and Group 3 (HAdV-11) preferentially interact with DSG2, but can use CD46 in the absence of DSG2 (72). DSG2 is a calcium-binding transmembrane glycoprotein in the desmosomes of epithelial junctions, which is widely distributed in the airway, gastrointestinal, and urinary tracts. DSG2 is also present in nonepithelial tissues among hematopoietic cells, dendritic cells, and cardiac muscle.
Animal models
Human adenovirus types of species B cannot infect rodent cells. Wang and colleagues (73) therefore generated transgenic mice containing the human DSG2 locus. These mice expressed human DSG2 (hDSG2) at a level and in a pattern similar to that found in humans and nonhuman primates. After intranasal application, efficient transduction of bronchial and alveolar epithelial cells was detected in hDSG2-transgenic mice. These hDSG2-transgenic mice are potentially an appropriate model with which to study: (I) the tropism of HAdVs, vectors or proteins that use hDSG2 as a receptor, (II) antiviral drugs or vaccines that interfere with Ad infection, and (III) certain downstream effects of the Ad3-hDSG2 interaction, including the potential toxicity associated with transient epithelial junction opening. As human adenoviruses do not replicate in mice, hDSG2-transgenic mice have limitations as a model to study adenovirus pathology, it may however be helpful in the study of HRRF.
While Syrian hamsters can support HAdV-C5 replication to some degree, it remains to be investigated whether hDSG2-transgenic hamsters will be adequate to study HAdV-B replication. Pathology in permissive syrian hamsters after infection with species C human adenovirus (HAdV-C) is the result of virus replication: HAdV-C6 replicates more and causes more pathology than HAdV-C5 (74). In another study, HAdV-C5-infected STAT2 knockout hamsters demonstrated an accentuated pathology compared with the wild type control animals, and the virus load in the organs of STAT2 knockout animals was 100- to 1,000-fold higher than that of wild-type hamsters. The adaptive immune response to adenovirus is not adversely affected in STAT2 knockout hamsters (69,75,76). Several studies have found that HAdV-5 can infect pigs, replicate in pig cells and cause lung disease, which is similar to the symptoms of humans infected by adenovirus (71). Recently, our team found that HAdV-3, -7, -14 and -55 could infect tree shrew cells, replicate efficiently, and produce infectious viral particles (unpublished data). Tree shrew (Tupaia belangeri) is a newly developed animal model for human diseases because of their close relationship to primates. This animal can be successfully infected with many human pathogenic viruses and has been widely used in research into oncology, endocrinology, neurology and ophthalmology (77).
Fine et al. identified FAM111A as an SV40 host range restriction and adenovirus helper factor in monkey cells. Depletion of FAM111A increased adenovirus replication in restrictive African green monkey kidney cells (CV-1) (78). Mutations in the viral gene encoding the DNA-binding protein allow HAdV-2 and -5 to express late genes and replicate in monkey cells and rhesus macaques across host restriction (79,80).
Adenovirus vaccine production
Cell lines
In the past, formalin-inactivated adenovirus vaccines were produced in primary monkey kidney cells but this raised problems associated with oncogenic potency and contamination with simian viruses. Instead, a technique to produce selective intestinal infection by feeding adenovirus in enteric-coated capsules was developed. Initially, the adenovirus strains were cultured in human embryonic kidney tissue, which was found to be unsuitable for large-scale vaccine production because of the possible contamination with hepatitis virus and other human pathogens. Some human diploid cell lines have been used in the production of current vaccines, such as WI-38 which was isolated in the 1960s. The current adenovirus type 4 and 7 vaccine approved for use in US military populations was produced by Teva Pharmaceuticals in WI-38 cells. In addition, the Vero cell line, which originated from the kidney of an African green monkey, has been used for vaccine production (42-44,81,82). Woods and coworkers demonstrated that the A549 cell line, a human lung carcinoma continuous cell line, was efficient and economical for the recovery of most adenovirus types (83). When 45 isolates of adenovirus were tested, 96% were detected in A549 cells, 62% were detected in HEp-2 cells, 38% were detected in MRC-5/WI-38 cells, and 31% were detected in PMK cells. The A549 cell line is therefore suitable for the production of adenovirus, but is not recommended for adenovirus vaccine production due to potential carcinogenic factors.
Purification technology
Currently, adenovirus manufacturing methods rely on well-established cell culture technologies to increase the yield and to reduce the overall manufacturing cost, such as cultivation at high cell densities and continuous downstream processing. Burova and colleagues summarized that the initial adenovirus purification strategy exploited the estimated density of hydrated adenovirus particles, which allowed for viral purification by cesium chloride (CsCl) density gradient ultracentrifugation (84). The main advantage of this method is the efficient separation of the recombinant viral particles from defective virions and cellular debris. However, the classical procedure involves two rounds of ultracentrifugation on a CsCl gradient and dialysis to remove the CsCl, consequently, the biological activity of the adenovirus could diminish or disappear during the purification. Ugai and coworkers therefore evaluated a rapid combination method, which included one round of ultracentrifugation on a CsCl gradient and tangential flow filtration, without significant loss of biological activity (85). Although the classical method of adenovirus purification by density gradient centrifugation is effective on a small scale, its main disadvantage is the limited capacity of laboratory centrifuges, restricting large-scale applications in large animal models and clinical trials. In addition, extensive dialysis of viral preparations is required due to CsCl toxicity. Other disadvantages of this method include variable quality of virus preparations, significant loss of infectivity and aggregation during storage.
To overcome the disadvantages of the classical purification method, column chromatography has been found to be the most powerful and versatile method for large-scale HAdV purification (Table 4). The yield, purity and biological potency of the final viral product resulting from chromatography-based purification schemes surpass those of conventional CsCl purification. The modes of chromatography applicable to these viruses include ion exchange, affinity, gel filtration and hydrophobic interaction chromatography. A series of optimized chromatographic steps is required for obtaining virus of high yield and purity. Two-step purification protocols, including two chromatographic steps or a combination of chromatography with ultracentrifugation/filtration, are optimal for HAdV vectors. Because many chromatographic elution buffers used for HAdV purification procedures are not suitable for in vivo manipulations, additional purification steps such as dialysis or concentration may be necessary. In the two-step chromatographic purification protocol, the first step employs column chromatography to capture adenoviruses following cell lysis, and the second step removes impurities such as cellular proteins and bacterial endotoxin. Chromatography is effectively applied in large-scale adenovirus purification when used alone or in combination with other technologies. Virus recovery from an anion-exchange column used as a first separation step can be as high as 99%. The virus purification procedure is completed by immobilized metal ion affinity chromatography (IMAC) and the overall virus yield and infectivity are superior to those obtained by CsCl gradient purification. In several reports, a number of anion-exchange adsorbents were evaluated for their performance in adenovirus particle purification (86,87).

Full table
Adjuvant
Vaccine development has traditionally focused on whole organism vaccines, either live attenuated or inactivated vaccines. VLP and sub-unit proteins offer a much safer alternative; however, they tend to be less immunogenic. Attempts have been made to increase immunogenicity with the addition of adjuvants, either immunostimulatory molecules or an antigen delivery system. Adjuvants have been traditionally used in the formulation of vaccines to (I) increase the response to a vaccine in the general population, (II) increase seroconversion rates in special populations (infants, the elderly, immunocompromised individuals), (III) facilitate the use of smaller doses of antigen, (IV) permit immunization with fewer doses of vaccine. The second reason for incorporating an adjuvant into a vaccine is to achieve qualitative alteration of the immune response: guiding the type of adaptive response to produce the most effective forms of immunity for each specific pathogen (88). Aluminum compounds (alum) are the most widely used adjuvants in veterinary and human vaccines. The most potent adjuvants for the formulation of vaccines include oil-based emulsions, mineral compounds, liposomes, bacterial products, ISCOMs and most recently used nanomaterials. An area of extreme interest has been the application of nanotechnology to vaccine development, which allows for antigens to be expressed on a particulate delivery system. One of the most exciting examples of nanovaccines are rationally designed protein nanoparticles (89). The majority of pharmaceutical based adjuvants currently being investigated are particulate based delivery systems, such as liposome formulations. As an adjuvant, liposomes have been shown to enhance immunity against the associated disease particularly when a cationic lipid is used within the formulation. Liposomes are readily to biodegradable in vivo and nontoxic and nonimmunogenic in animals. Chen and colleagues used pertussis vaccine as an adjuvant for the HAdV-3 subunit antigens and successfully enhanced the immunogenicity of the subunit antigens and increased the yield of antibodies to ensure long-lasting immunity (90). In particular, the liposome technology is ideal for combining protein antigen and adjuvant into an effective mucosal vaccine. Nanoparticle formulations using various types of liposomes convey strong promise for the successful development of the next generation of mucosal vaccines (91).
Conclusions
The prevalent adenovirus types leading to severe acute respiratory tract disease are thought to be HAdV-B3, B7, B4, B14 and B55; however, further studies may reveal some geographical and population-based differences. The live oral adenovirus vaccine has been shown to be safe and highly effective among US military personnel over several decades, with no evidence of its oncogenic capacity. However, a live vaccine based on a wild adenovirus strain should not be recommended for use in children or the general population. Therefore, innovative adenovirus vaccines, including inactivated, epitope-based, subunit and replication-defective adenovirus vaccines, should be considered. In fact, early studies conducted between 1956 and 1960 demonstrated that inactivated adenovirus vaccines were highly effective in preventing adenovirus disease. The limitations imposed by adenovirus receptors and HRRF mean that adenovirus species B cannot infect rodent cells and replicate in mice cells. It remains to be investigated whether hDSG2-transgenic hamsters will permit the study of HAdV-B replication. Currently, pigs and tree shrews are being investigated as potential adenovirus models.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (NSFC 31570163).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pavia AT. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis 2011;52:S284-9. [Crossref] [PubMed]
- Lenaerts L, De Clercq E, Naesens L. Clinical features and treatment of adenovirus infections. Rev Med Virol 2008;18:357-74. [Crossref] [PubMed]
- Sandkovsky U, Vargas L, Florescu DF. Adenovirus: Current epidemiology and emerging approaches to prevention and treatment. Curr Infect Dis Rep 2014;16:416. [Crossref] [PubMed]
- Seto D, Chodosh J, Brister JR, et al. Using the whole-genome sequence to characterize and name human adenoviruses. J Virol 2011;85:5701-2. [Crossref] [PubMed]
- Singh G, Robinson CM, Dehghan S, et al. Homologous recombination in E3 genes of human adenovirus species D. J Virol 2013;87:12481-8. [Crossref] [PubMed]
- Yoshitomi H, Sera N, Gonzalez G, et al. First isolation of a new type of human adenovirus (genotype 79), species Human mastadenovirus B (B2) from sewage water in Japan. J Med Virol 2017;89:1192-200. [Crossref] [PubMed]
- Wang Y, Li Y, Lu R, et al. Phylogenetic evidence for intratypic recombinant events in a novel human adenovirus C that causes severe acute respiratory infection in children. Sci Rep 2016;6:23014. [Crossref] [PubMed]
- Chen M, Zhu Z, Huang F, et al. Adenoviruses associated with acute respiratory diseases reported in Beijing from 2011 to 2013. PLoS One 2015;10. [Crossref] [PubMed]
- Guo L, Gonzalez R, Zhou H, et al. Detection of three human adenovirus species in adults with acute respiratory infection in China. Eur J Clin Microbiol Infect Dis 2012;31:1051-8. [Crossref] [PubMed]
- Gu L, Liu Z, Li X, et al. Severe community-acquired pneumonia caused by adenovirus type 11 in immunocompetent adults in Beijing. J Clin Virol 2012;54:295-301. [Crossref] [PubMed]
- Cao B, Huang GH, Pu ZH, et al. Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia. Chest 2014;145:79-86. [Crossref] [PubMed]
- Deng J, Qian Y, Zhao LQ, et al. Identification and typing of adenovirus from acute respiratory infections in pediatric patients in Beijing from 2003 to 2012. Bing Du Xue Bao 2013;29:615-20. [PubMed]
- Sun B, He H, Wang Z, et al. Emergent severe acute respiratory distress syndrome caused by adenovirus type 55 in immunocompetent adults in 2013: A prospective observational study. Crit Care 2014;18:456. [Crossref] [PubMed]
- Carr MJ, Kajon AE, Lu X, et al. Deaths associated with human adenovirus-14p1 infections, Europe, 2009–2010. Emerg Infect Dis 2011;17:1402-8. [Crossref] [PubMed]
- Mi Z, Butt AM, An X, et al. Genomic analysis of HAdV-B14 isolate from the outbreak of febrile respiratory infection in China. Genomics 2013;102:448-55. [Crossref] [PubMed]
- Metzgar D, Osuna M, Kajon AE, et al. Abrupt emergence of diverse species B adenoviruses at US military recruit training centers. J Infect Dis 2007;196:1465-73. [Crossref] [PubMed]
- Kajon AE, Lu X, Erdman DD, et al. Molecular epidemiology and brief history of emerging adenovirus 14-associated respiratory disease in the United States. J Infect Dis 2010;202:93-103. [Crossref] [PubMed]
- Lu QB, Tong YG, Wo Y, et al. Epidemiology of human adenovirus and molecular characterization of human adenovirus 55 in China, 2009–2012. Influenza Other Respir Viruses 2014;8:302-8. [Crossref] [PubMed]
- Radin JM, Hawksworth AW, Blair PJ, et al. Dramatic decline of respiratory illness among US military recruits after the renewed use of adenovirus vaccines. Clinical infectious diseases 2014;59:962-8. [Crossref] [PubMed]
- Hoke CH, Snyder CE. History of the restoration of adenovirus type 4 and type 7 vaccine, live oral (Adenovirus Vaccine) in the context of the Department of Defense acquisition system. Vaccine 2013;31:1623-32. [Crossref] [PubMed]
- Tabain I, Ljubin-Sternak S, Cepin-Bogovi J, et al. Adenovirus respiratory infections in hospitalized children: clinical findings in relation to species and serotypes. Pediatr Infect Dis J 2012;31:680-4. [Crossref] [PubMed]
- Dong YS. Unite and devote to the works of pediatric clinical virology. Zhonghua Er Ke Za Zhi 2005;43:1-2. [PubMed]
- Sun HQ, Zhang XX, Kuang XN, et al. Epidemiological analysis of 440 cases of respiratory adenovirus infections in children from the Suzhou area between 2006 and 2015. Zhongguo Dang Dai Er Ke Za Zhi 2017;19:34-8. [PubMed]
- Zhao S, Wan C, Ke C, et al. Re-emergent Human Adenovirus Genome Type 7d Caused an Acute Respiratory Disease Outbreak in Southern China After a Twenty-one Year Absence. Scientific Reports 2014;4:7365. [Crossref] [PubMed]
- Lai CY, Lee CJ, Lu CY, et al. Adenovirus serotype 3 and 7 infection with acute respiratory failure in children in Taiwan, 2010-2011. PLoS One 2013;8. [Crossref] [PubMed]
- Chen Y, Liu F, Wang C, et al. Molecular Identification and Epidemiological Features of Human Adenoviruses Associated with Acute Respiratory Infections in Hospitalized Children in Southern China, 2012-2013. PLoS One 2016;11. [Crossref] [PubMed]
- Huang G, Yu D, Zhu Z, et al. Outbreak of febrile respiratory illness associated with human adenovirus type 14p1 in Gansu Province, China. Influenza Other Respir Viruses 2013;7:1048-54. [Crossref] [PubMed]
- Liu L, Qian Y, Zhang Y, et al. Adenoviruses associated with acute diarrhea in children in Beijing, China. PLoS One 2014;9. [Crossref] [PubMed]
- Zhang QL, Wang HB, Wang YL, et al. Genotypes of adenoviruses in infants and young children with diarrhea. Zhongguo Dang Dai Er Ke Za Zhi 2016;18:718-20. [PubMed]
- Centers for Disease Control and Prevention (CDC). Acute respiratory disease associated with adenovirus serotype 14-four states, 2006-2007. MMWR Morb Mortal Wkly Rep 2007;56:1181-4. [PubMed]
- Vento TJ, Prakash V, Murray CK, et al. Pneumonia in military trainees: a comparison study based on adenovirus serotype 14 infection. J Infect Dis 2011;203:1388-95. [Crossref] [PubMed]
- Potter RN, Cantrell JA, Mallak CT, et al. Adenovirus-associated deaths in US military during post vaccination period, 1999–2010. Emerg Infect Dis 2012;18:507-9. [Crossref] [PubMed]
- Yu P, Ma C, Nawaz M, et al. Outbreak of acute respiratory disease caused by human adenovirus type 7 in a military training camp in Shaanxi, China. Microbiol Immunol 2013;57:553-60. [Crossref] [PubMed]
- Cheng J, Qi X, Chen D, et al. Epidemiology and transmission characteristics of human adenovirus type 7 caused acute respiratory disease outbreak in military trainees in East China. Am J Transl Res 2016;8:2331-42. [PubMed]
- Kajon AE, Dickson LM, Metzgar D, et al. Outbreak of febrile respiratory illness associated with adenovirus 11a infection in a Singapore military training cAMP. J Clin Microbiol 2010;48:1438-41. [Crossref] [PubMed]
- Chmielewicz B, Benzler J, Pauli G, et al. Respiratory disease caused by a species B2 adenovirus in a military camp in Turkey. J Med Virol 2005;77:232-7. [Crossref] [PubMed]
- Walsh MP, Seto J, Jones MS, et al. Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J Clin Microbiol 2010;48:991-3. [Crossref] [PubMed]
- Li X, Kong M, Su X, et al. An outbreak of acute respiratory disease in China caused by human adenovirus type B55 in a physical training facility. Int J Infect Dis 2014;28:117-22. [Crossref] [PubMed]
- Zhang Q, Seto D, Cao B, et al. Genome sequence of human adenovirus type 55, a re-emergent acute respiratory disease pathogen in China. J Virol 2012;86:12441-2. [Crossref] [PubMed]
- Lion T. Adenovirus Infections in Immunocompetent and Immunocompromised Patients. Clin Microbiol Rev 2014;27:441-62. [Crossref] [PubMed]
- Matthes-Martin S, Boztug H, Lion T. Diagnosis and treatment of adenovirus infection in immunocompromised patients. Expert Rev Anti Infect Ther 2013;11:1017. [Crossref] [PubMed]
- Hilleman MR. Efficacy of and Indications for Use of Adenovirus Vaccine. Am J Public Health Nations Health 1958;48:153-8. [Crossref] [PubMed]
- Top FH Jr, Grossman RA, Bartelloni PJ, et al. Immunization with live types 7 and 4 adenovirus vaccines. I. Safety, infectivity, antigenicity, and potency of adenovirus type 7 vaccine in humans. J Infect Dis 1971;124:148-54. [Crossref] [PubMed]
- Top FH Jr, Buescher EL, Bancroft WH, et al. Immunization with live types 7 and 4 adenovirus vaccines. II. Antibody response and protective effect against acute respiratory disease due to adenovirus type 7. J Infect Dis 1971;124:155-60. [Crossref] [PubMed]
- Hoke CH Jr, Snyder CE Jr. History of the restoration of adenovirus type 4 and type 7 vaccine, live oral (Adenovirus Vaccine) in the context of the Department of Defense acquisition system. Vaccine 2013;31:1623-32. [Crossref] [PubMed]
- Gray GC, Goswami PR, Malasig MD, et al. Adult adenovirus infections: loss of orphaned vaccines precipitates military respiratory disease epidemics. For the Adenovirus Surveillance Group. Clin Infect Dis 2000;31:663-70. [Crossref] [PubMed]
- Potter RN, Cantrell JA, Mallak CT, et al. Adenovirus-associated deaths in US military during post vaccination period, 1999–2010. Emerg Infect Dis 2012;18:507-9. [Crossref] [PubMed]
- Radin JM, Hawksworth AW, Blair PJ, et al. Dramatic decline of respiratory illness among US military recruits after the renewed use of adenovirus vaccines. Clinical infectious diseases 2014;59:962-8. [Crossref] [PubMed]
- Anderson BD, Ma M, Ma M, et al. Emerging adenovirus threats: should China develop a vaccine-oriented prevention strategy? Zhonghua Yu Fang Yi Xue Za Zhi 2014;48:1030-4. [PubMed]
- Meiklejohn G. Present and future of inactivated virus vaccines. Am Rev Respir Dis 1963;88:372-8. [PubMed]
- Chanock RM, Ludwig W, Heubner RJ, et al. Immunization by selective infection with type 4 adenovirus grown in human diploid tissue cultures. I. Safety and lack of oncogenicity and tests for potency in volunteers. JAMA 1966;195:445-52. [Crossref] [PubMed]
- Truffelli GT, Timm EA, Beardmore WB, et al. Inactivation of Adenovirus and Simian Virus 40 Tumorigenicity in Hamsters by Vaccine Processing Methods. Appl Microbiol 1967;15:516-27. [PubMed]
- Sumida SM, Truitt DM, Lemckert AA, et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol 2005;174:7179-85. [Crossref] [PubMed]
- Bradley RR, Lynch DM, Iampietro MJ, et al. Adenovirus serotype 5 neutralizing antibodies target both hexon and fiber following vaccination and natural infection. J Virol 2012;86:625-9. [Crossref] [PubMed]
- Yu B, Dong J, Wang C, et al. Characteristics of neutralizing antibodies to adenovirus capsid proteins in human and animal sera. Virology 2013;437:118-23. [Crossref] [PubMed]
- Tian X, Su X, Li H, et al. Construction and characterization of human adenovirus serotype 3 packaged by serotype 7 hexon. Virus Res 2011;160:214-20. [Crossref] [PubMed]
- Yuan X, Qu Z, Wu X, et al. Molecular modeling and epitopes mapping of human adenovirus type 3 hexon protein. Vaccine 2009;27:5103-10. [Crossref] [PubMed]
- Yuan XH, Wang YC, Jin WJ, et al. Structure-based high-throughput epitope analysis of hexon proteins in B and C species human adenoviruses (HAdVs). PLoS One 2012;7. [Crossref] [PubMed]
- Qiu H, Li X, Tian X, et al. Serotype-specific neutralizing antibody epitopes of human adenovirus type 3 (HAdv-3) and HAdV-7 reside in multiple hexon hypervariable regions. J Virol 2012;86:7964-75. [Crossref] [PubMed]
- Yu B, Dong J, Wang C, et al. Trimeric knob protein specifically distinguishes neutralizing antibodies to different human adenovirus species: potential application for adenovirus seroepidemiology. J Gen Virol 2014;95:1564-73. [Crossref] [PubMed]
- Lang S, Wang L, Wang Z, et al. Localization of neutralization epitopes on adenovirus fiber knob from species C. J Gen Virol 2016;97:955-62. [Crossref] [PubMed]
- Tian X, Ma Q, Jiang Z, et al. Identification and Application of Neutralizing Epitopes of Human Adenovirus Type 55 Hexon Protein. Viruses 2015;7:5632-42. [Crossref] [PubMed]
- Ma Q, Tian X, Jiang Z, et al. Neutralizing epitopes mapping of human adenovirus type 14 hexon. Vaccine 2015;33:6659-65. [Crossref] [PubMed]
- Liu M, Tian X, Li X, et al. Generation of Neutralizing Monoclonal Antibodies against a Conformational Epitope of Human Adenovirus Type 7 (HAdv-7) Incorporated in Capsid Encoded in a HAdv-3-Based Vector. PLoS One 2014;9. [Crossref] [PubMed]
- Cheng CY, Gilson T, Wimmer P, et al. Role of E1B55K in E4orf6/E1B55K E3 ligase complexes formed by different human adenovirus serotypes. J Virol 2013;87:6232-45. [Crossref] [PubMed]
- Su X, Tian X, Jiang Z, et al. Human Adenovirus Serotype 3 Vector Packaged by a Rare Serotype 14 Hexon. PLoS One 2016;11. [Crossref] [PubMed]
- Gupta A, Ahmed KA, Ayalew LE, et al. Immunogenicity and protective efficacy of virus-like particles and recombinant fiber proteins in broiler-breeder vaccination against fowl adenovirus (FAdV)-8b. Vaccine 2017;35:2716-22. [Crossref] [PubMed]
- Prince GA, Porter DD, Jenson AB, et al. Pathogenesis of adenovirus type 5 pneumonia in cotton rats (Sigmodon hispidus). J Virol 1993;67:101-11. [PubMed]
- Wold WS, Toth K. Chapter three--Syrian hamster as an animal model to study oncolytic adenoviruses and to evaluate the efficacy of antiviral compounds. Adv Cancer Res 2012;115:69-92. [Crossref] [PubMed]
- Romanowski EG, Gordon YJ, Araullo-Cruz T, et al. The antiviral resistance and replication of cidofovir-resistant adenovirus variants in the New Zealand White rabbit ocular model. Invest Ophthalmol Vis Sci 2001;42:1812-5. [PubMed]
- Jogler C, Hoffmann D, Theegarten D, et al. Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species. J Virol 2006;80:3549-58. [Crossref] [PubMed]
- Wang H, Li ZY, Liu Y, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med 2011;17:96-104. [Crossref] [PubMed]
- Wang H, Beyer I, Persson J, et al. A new human DSG2-transgenic mouse model for studying the tropism and pathology of human adenoviruses. J Virol 2012;86:6286-302. [Crossref] [PubMed]
- Tollefson AE, Ying B, Spencer JF, et al. Pathology in Permissive Syrian Hamsters after Infection with Species C Human Adenovirus (HAdV-C) Is the Result of Virus Replication: HAdV-C6 Replicates More and Causes More Pathology than HAdV-C5. J Virol 2017;91. [Crossref] [PubMed]
- Thomas MA, Spencer JF, La Regina MC, et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res 2006;66:1270-6. [Crossref] [PubMed]
- Toth K, Lee SR, Ying B, et al. STAT2 Knockout Syrian Hamsters Support Enhanced Replication and Pathogenicity of Human Adenovirus, Revealing an Important Role of Type I Interferon Response in Viral Control. PLoS pathogens 2015;11. [Crossref] [PubMed]
- Fan Y, Huang ZY, Cao CC, et al. Genome of the Chinese tree shrew. Nat Commun 2013;4:1426. [Crossref] [PubMed]
- Fine DA, Rozenblatt-Rosen O, Padi M, et al. Identification of FAM111A as an SV40 host range restriction and adenovirus helper factor. PLoS Pathog 2012;8. [Crossref] [PubMed]
- Qureshi H, Genescà M, Fritts L, et al. Infection with host-range mutant adenovirus 5 suppresses innate immunity and induces systemic CD4+ T cell activation in rhesus macaques. PLoS One 2014;9. [Crossref] [PubMed]
- Klessig DF, Grodzicker T. Mutations that allow human Ad2 and Ad5 to express late genes in monkey cells map in the viral gene encoding the 72K DNA binding protein. Cell 1979;17:957-66. [Crossref] [PubMed]
- Jordan I, Sandig V. Matrix and Backstage: Cellular Substrates for Viral Vaccines. Viruses 2014;6:1672-700. [Crossref] [PubMed]
- Tint H, Stone JL, Minecci LC, et al. Type 4 adenovirus vaccine, live, prepared in human diploid cell system for oral administration. Prog Immunobiol Stand 1967;3:113-22. [PubMed]
- Woods GL, Young A. Use of A-549 cells in a clinical virology laboratory. J Clin Microbiol 1988;26:1026-8. [PubMed]
- Burova E, Ioffe E. Chromatographic purification of recombinant adenoviral and adeno-associated viral vectors: methods and implications. Gene Ther 2005;12:S5-17. [Crossref] [PubMed]
- Ugai H, Yamasaki T, Hirose M, et al. Purification of infectious adenovirus in two hours by ultracentrifugation and tangential flow filtration. Biochem Biophys Res Commun 2005;331:1053-60. [Crossref] [PubMed]
- Morenweiser R. Downstream processing of viral vectors and vaccines. Gene Ther 2005;12:S103-10. [Crossref] [PubMed]
- Kallel H, Kamen AA. Large-scale adenovirus and poxvirus-vectored vaccine manufacturing to enable clinical trials. Biotechnol J 2015;10:741-7. [Crossref] [PubMed]
- Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity 2010;33:492-503. [Crossref] [PubMed]
- Karch CP, Burkhard P. Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem Pharmacol 2016;120:1-14. [Crossref] [PubMed]
- Kramp WJ, Six HR, Drake S, et al. Liposomal enhancement of the immunogenicity of adenovirus type 5 hexon and fiber vaccines. Infect Immun 1979;25:771-3. [PubMed]
- Bernasconi V, Norling K, Bally M, et al. Mucosal Vaccine Development Based on Liposome Technology. J Immunol Res 2016;2016. [Crossref] [PubMed]


