Prophylactic continuous positive airway pressure after pulmonary lobectomy: a randomized controlled trial
Introduction
Lung resection with curative intent is indicated for patients with non-small-cell lung cancer at early stages that are fit to tolerate surgery. In these cases, open or thoracoscopic pulmonary lobectomy is the treatment of choice (1). Despite advances in perioperative care and surgical techniques, patients undergoing pulmonary lobectomy are still at high risk of developing postoperative complications (2,3). Actually, an alteration of the respiratory function has been observed after thoracic surgery; such alteration is characterized by a restrictive pattern, with a reduction of lung flows and volumes that can contribute to the onset of postoperative complications along with the presence of variable degrees of hypoxia, diaphragm dysfunction, impaired airway clearance and pain (4). In the clinical practice, the mentioned physiopathological alterations could result in atelectasis, pulmonary infections and cardiac disorders that are common after pulmonary lobectomy and play an important role in the mortality rate, hospital costs and patients’ comfort (5,6). Among the postoperative interventions aimed to reduce complications, aside from adequate pain management and standard respiratory physiotherapy, positive airway pressure delivered by a noninvasive interface—continuous positive airway pressure (CPAP) or non-invasive ventilation (NIV)—seems to have a positive impact (7). The capability of the positive airway pressure to antagonize the postoperative restrictive pattern and to improve pulmonary function have been documented; in addition, it has also been demonstrated how NIV is effective in reducing the need for intubation and consequently the mortality rate in the postoperative period in patients with acute respiratory failure (8-11). If this functional improvement has any significant clinical benefit on the postoperative course after pulmonary lobectomy remains to be understood (12). In this uncertainty, we conducted a prospective randomized controlled clinical trial to test the hypothesis whether prophylactic application of CPAP following pulmonary lobectomy can reduce postoperative complications.
Methods
Patients with clinical stage I non-small cell lung cancer electively scheduled for pulmonary lobectomy were eligible to participate in the study. Exclusion criteria were: age <18 or >78 years, no consent, diagnosis of obstructive sleep apnea syndrome, evidence of lack of adherence to treatment verified during the preoperative education, physical disabilities limiting participation to a standard physiotherapy treatment, mini-mental state examination <20 and postoperative mechanical ventilation >24 hours.
The local ethics committee approved the protocol (2369/2012), and written informed consent was obtained from the patients before surgery. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki and registered on line at ISRCTN with the number 13454737.
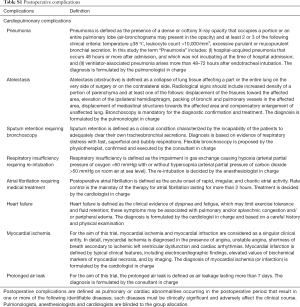
The study was designed as a prospective, partially blind, randomized, controlled, parallel trial; the study setting involved one university hospital and one tertiary hospital in the same city. The primary endpoint of the trial was the rate of postoperative complications; such composite parameter was defined as the occurrence of postoperative cardiopulmonary adverse events (which included atelectasis, pneumonia, sputum retention requiring bronchoscopy, respiratory insufficiency requiring re-intubation, atrial fibrillation requiring medical treatment, heart failure and myocardial ischemia) and prolonged air leak (>7 days). Secondary endpoints were early cardiorespiratory parameters, length of hospital stay and 30-day mortality (discharging rules and postoperative complication definitions are reported in the supplementary material).
Preoperative functional study comprised pulmonary function tests (PFTs), diffusion lung capacity for CO, 6-minute walking test and arterial blood gas analysis; ventilation/perfusion lung scan and/or cardiopulmonary exercise test were done in selected cases. From 10 to 3 days prior surgery, eligible patients were trained for the use of CPAP interface.
Surgical procedures consisted in pulmonary lobectomy with systematic lymphadenectomy preferentially via standardized three-port anterior approach video-assisted thoracic surgery (VATS) (13); muscle-sparing lateral thoracotomy was planned in case of cancers located close to vascular structures and/or calcified hilar lymph-nodes. VATS lobectomies requiring conversion to open surgery were considered as “open surgery”. All patients received intercostal block with levobupivacaine as preventive analgesia. Postoperative pain management included systemic analgesia with morphine in the first 48 hours followed by acetaminophen/codeine phosphate administration; ketorolac was used as rescue medication. Postoperative physiotherapeutic treatment was comprehensive of early mobilization, maintenance of the seated position, deambulation and assisted cough during the three days after surgery according to a standardized protocol.
After surgery, the patient allocation was achieved using randomized numbers generated by a computer. Both groups received standard postoperative pain management and physiotherapy as described above. The study group, in addition, received CPAP (Stellar 150, ResMed Ltd., Bella Vista, NSW, Australia) delivered via oronasal mask (Mirage Quattro™ Full Face Mask, ResMed Ltd, Bella Vista, NSW, Australia) starting from the first day after surgery. The Table 1 describes physiotherapeutic and intervention protocols.

Full table
In addition to the clinical parameters useful for the primary endpoint, study measurements included index of prolonged air leak (IPAL) calculation (14), PFTs on the 1st and 4th postoperative day (POD), arterial blood gas analysis in room air on the 1st and 4th POD, chest X-ray on 1st and 3rd POD, 6-minute walking test on 5th POD, daily recording of air leakage with digital device, days of permanence of the chest drain, numerical rating scale pain assessment and length of hospital stay. Operators in charge for PFTs, arterial blood gas analysis, 6-minute walking test and chest X-ray were blinded to patients’ allocation having no access to patients’ charts. The economic calculation was limited to the cost of the physiotherapist, the mask and at the expense of a hospitalization. Supplementary section includes the discharging criteria and postoperative complications definitions (Table S1).
Full table
Sample size calculation considered the three years retrospective analysis of postoperative complications after pulmonary lobectomy in both hospitals participating in the trial, as well as a large national report published shortly before the study plan (15); finally, we fixed as 45% the rate of complications in the control group and we postulated that the reduction of 22% could have a clinical impact. Therefore, setting Alpha at 0.05, Beta at 0.20 and 1:1 as group proportion, the sample size resulted of 162 patients. Being the end-point rather short, we did not increase the sample size for possible patients lost to follow-up. The MedCalc 14.8 software was used for statistical analysis. Data are shown as the mean ± standard deviation (SD) or median with 95% confidence interval (CI). To compare the categorical variables, the chi-square test or Fisher’s exact test were used; continuous variables were compared with the Mann-Whitney test or the t-test. Analysis of variance for repeated measurements (repeated-measure ANOVA) was used to compare measurements between longitudinal groups. Logistic regression was used to test the significance of each variable; the model was built in two steps: first, clinically relevant variables were included in a univariate model, and second, a stepwise modeling approach was used introducing all variables with p value lower than 0.05 in the first step. Tests were two-sided and P values at 0.05 or less were considered to be statistically significant.
Results
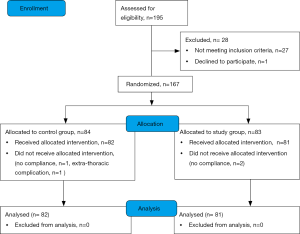
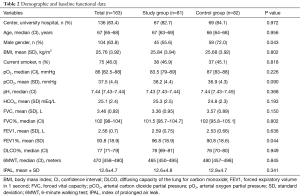
A total of 195 patients were considered for the trial; 167 were enrolled in 40 months (Figure S1 in the supplementary material shows the patients flow chart). After the appropriate selection 163 patients were considered for the descriptive and inferential analysis: 82 patients constituted the control group and 81 the study group (feasibility metrics are reported in supplementary material). There were no statistical differences regarding demographic data between groups except the lower percentage of male gender in the study group. Also, the baseline functional parameters were comparable between groups but the FEV1, expressed in percentage of the predicted value, was significantly lower in the control group; the Table 2 shows demographic and baseline functional data. Surgical procedures were similar between the groups (additional data are presented in the supplementary section). The CPAP treatment was generally fairly tolerated; 11.5 (SD 3.9) hours was the mean time of actual CPAP administration. Adverse effects related to CPAP administration were not observed in the study population.
Full table
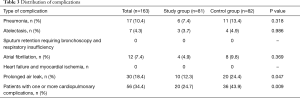
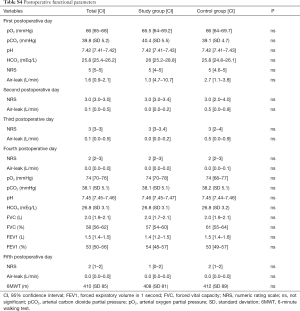
The rate of patients with one or more postoperative complications was significantly lower in the study group (24.7% vs. 43.9%; P=0.015); the Table 3 describes the distribution of all the complications recorded. The relative risk reduction resulted 0.5624 (CI: 0.3578–0.8840; P=0.0126) and the number needed to treat was 5.205 [CI: 2.986 (benefit) to 20.280 (benefit)]. The analysis of the secondary end-points underlined shorter hospital stay in the study group (6 vs. 7 days; P=0.031) while none of the groups had 30-day mortality. None of the functional parameters recorded during the early postoperative period resulted statistically different between the two groups. The analysis of variance for repeated measures demonstrated that all pulmonary function indexes significantly decreased their values in the early postoperative period; there were found any differences between the groups.
Full table
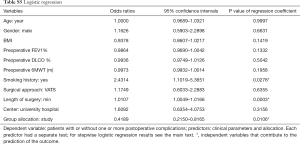
Analyzing with logistic regression test the impact of the variables, which potentially could impact on postoperative complications, three items resulted significant: group allocation, length of surgery and smoking habit; on the contrary, age, surgical approach, body mass index, diffusion lung capacity for CO, 6-minute walking test, FEV1% of predicted and hospital distribution did not predict postoperative complications. Entering the significant items into the stepwise logistic regression model, the three predictive parameters were still related with postoperative complications: belonging to the study group [odd ratio (OR): 0.3026, CI: 0.1389–0.6591; P=0.0026], smoke habits OR: 2.5835, CI: 1.0331–6.4610; P=0.0424) and the length of surgery in minutes (OR: 1.0102, CI: 1.0042–1.0163; P=0.0009).
The time spent on each patient by the physiotherapist was about three hours, while masks were re-used twenty times: (€25.00×3) + (€200.00/20) = €85.00 per patients; the estimated cost of a day’s stay in the thoracic surgery ward, including medication, is €787.00.
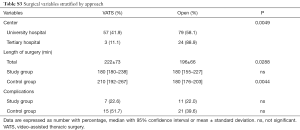
Supplementary section includes: surgical variables stratified by group (Table S2) and by approach (Table S3); postoperative functional parameters (Table S4); logistic regression results (Table S5) and feasibility metrics (Table S6).

Full table
Full table
Full table
Full table

Full table
Discussion
Originally utilized for acute exacerbations of chronic obstructive pulmonary disease or cardiogenic pulmonary edema, NIV was also applied to treat acute respiratory failure after major surgery (16-18). The prophylactic use of positive airway pressure is increasingly employed after major abdominal operations; a Cochrane systematic review published in 2014 revealed that prophylactic administration of CPAP after major abdominal surgery could reduce postoperative atelectasis, pneumonia and reintubation, but the quality of evidences was very low (19).
In the late nineties, the prophylactic use of NIV after thoracic surgery was tested by Aguilò and collaborators with a little prospective randomized study reporting positive results in gas exchanges (20). The Centre Hospitalier et Universitaire de Nice, France, conducted a randomize trial in 2007; thirty-two patients with lung resection were enrolled and the Authors concluded that NIV improved the arterial blood gas values (10). Ludwig and collaborators published a randomized study in 2011; CPAP was administered to 135 patients who received different types of lung resections (21). The complications rate resulted similar between the study group and the control group. A further randomized trial was published by Barbagallo and colleagues in 2012: two short cycles of CPAP were administered during the first postoperative day after pulmonary lobectomy (22). The Authors demonstrated better oxygenation in the study group immediately after the CPAP administration. A similar study was conducted in Madrid; 110 patients were enrolled and the study group received 6 hours of CPAP in the first postoperative day after various types of lung resections (11). The Authors concluded that the prophylactic use of CPAP improved oxygenation. The mentioned trials were included in a meta-analysis published in 2015; the Authors cannot demonstrate any positive effect of NIV or CPAP on pulmonary complications rate after lung resection, but small sample size and low frequency of events seriously affected the quality of the evidences; therefore, trials with appropriate characteristics were advocated (12).
The present trial was designed to minimize possible risk of bias: a computer random number generator was used and operators dedicated to assess outcomes were blinded for group allocation. Heterogeneity was also limited enrolling only patients requiring pulmonary lobectomy for stage I non-small cell lung cancer. It is well known that single-center studies have a potentially limited external validity essentially related to local resources and case-mix. Therefore, we decided to involve two large hospitals in the same city; the main reason for limiting the center numerosity was the intention to minimize possible undetectable protocol violations and to abate costs related to organization, quality control and data management.
Intervention protocols reported in the scientific literature were extremely variable in terms of length, frequency and ventilatory mode (12); we chose the CPAP ventilator because this type of positive airway pressure seems to be the first and simple step in NIV. On the other hand, we decided to plan a consistent time frame in the daily application of CPAP (6 hours) for the first three postoperative days. In the absence of any evidence on which type of CPAP protocol is preferable in the postoperative period, we assumed that an intensive program could provide measurable results. The primary end-point was chosen in all the cardiopulmonary complications that may affect the postoperative course rather than select specific item as atelectasis or pneumonia. Prolonged air leak was added to cardiopulmonary complications to create an aggregate parameter. This choice responded to the intention to set up a trial with clinical relevance instead of a mainly speculative study. The decision to include in the trial both open and thoracoscopic approach to pulmonary lobectomy was on the same line: it was clear that two approaches added a confounder but the population resulted closer to the real life in a thoracic department.
The population resulted homogeneous between the study and the control group in baseline characteristics except for gender and FEV1 percent of predicted. We do not think that gender could affect the main outcome, but the low FEV1 in the control group may represent a bias. It is true that randomization guarantees unbiased patients allocation, but not “automatically” balanced baseline characteristics. To be pragmatic, the practice of comparing baseline characteristics with statistical tests is disapproved by methodologists (23,24) because randomization warrants that groups’ difference is exclusively due to chance. Baseline characteristics check with statistical test has been depicted as unnecessary and sometime even harmful (23,25). In our specific case, supported by the logistic regression test that denies FEV1% to be a predictor of the main outcome, we consider the unbalanced baseline FEV1% as negligible. Operative parameters resulted uniform between the groups in terms of types of lobectomy, surgical approach, side and length of surgery contributing to the homogeneity of the population.
Considering that the intervention protocol was rather demanding for the patients, we positively judged that the CPAP was applied for 11.5 hours in mean, reaching the 64% of the planned time. Actually, despite advances in both machine and masks, the adherence rate to CPAP generally ranges from 30% to 60% (12). We believe that adequate preoperative education is fundamental to optimize the adherence. We observed neither severe skin lesions nor gastric distension during CPAP administration.
The rate of patients with postoperative complications resulted significantly lower in the study group; moreover, the prophylactic use of CPAP after pulmonary lobectomy is a predictor of a low postoperative cardiopulmonary complications rate in the stepwise logistic regression analysis. We are aware that this is the first time that this kind of message is prompted; we believe that these positive results were related to protocol characteristics, mainly for the frequency and duration of the intervention. We are not able to describe the physiological mechanisms underlying the therapeutic success of the CPAP administration because the trial was designed to return a merely clinically result; we can only argue that maintaining good oxygenation and adequate parenchymal expansion, at least for the CPAP period, may have prevented the occurrence of conditions favoring the onset of postoperative complications.
Interestingly, the length of surgery was a significant predictor of high postoperative complications rate in the stepwise logistic regression test: each additional minute increases the risk of complication of 1%. It is easy to understand how a longer operation can be at the same time more complex and therefore more exposed to postoperative complications. On the other hand, it is well known that unilateral ventilation can cause damage to the recumbent lung. Not surprisingly, the last factor that influenced the complication rate in the stepwise logistic regression analysis was the smoke habit: patients, who have smoked or are an active smoker, have the risk of complication increase of 150%. It is remarkable that the surgical approach (open versus VATS) did not impact on the postoperative complication rate; considering that it was found a trend favorable to VATS, it is possible that the present trial was underpowered to detect a significant difference.
The lack of events prevented us from analyzing 30-day mortality; on the contrary, the length of hospital stay was significantly lower in the study group. This positive result is in countertendency with the literature, considering that only one trial reported a significant reduction in hospital stay (10). On the other hand, our “statistically significant result” consisted in one day reduction of the hospital stay, but if this day has any “clinical significance” requires a reflection, especially because the patient discharge frequently implies social considerations besides the clinical evaluation. Anyway, from a strictly economic point of view the application of the CPAP resulted in a saving of €702.00 per patient.
Among secondary outcomes, we failed to demonstrate that CPAP could improve pulmonary functional tests in our subset of patients. Some trials, focused on the improvement of arterial blood gases and lung volumes immediately after CPAP administration, had positive results (10,11,22). To be honest, we did not expect that the CPAP could modify functional parameters when these were measured at consistent distance from the intervention as we did; instead, we believe that the improvement in gas exchange and lung ventilation obtained during the CPAP administration could create the condition capable to prevent postoperative complications.
One of the concerns about the use of CPAP after pulmonary resection is the possible increment of air leak in the postoperative period. In contrast, we did not record any difference in air leak between the groups; indeed, a tendency towards a protective effect was evident. The same result was achieved by other authors (22).
The present trial has some limitations. We enrolled patients who received the pulmonary lobectomy with thoracotomy or VATS access, even though the multivariate statistical analysis denies any impact on the primary outcome, it remains a possible enrolment bias. As mentioned above, baseline FEV1% of predicted was significantly higher in the study group, despite the methodological objections previously expressed and the fact that FEV1 does not predict complications in logistic regression, the unbalanced FEV1 could be suspected to be a bias. It is possible that a selection bias affected the whole population, considering the index for prolonged air leakage resulted particularly high in both groups. Despite the length of surgery was a regressor for postoperative complications, the trail was not designed to capture the variables affecting the length of surgery. Moreover, this study cannot establish a time threshold beyond which the CPAP shows its advantage.
In conclusion, the present trial demonstrated that prophylactic application of CPAP during the postoperative period after pulmonary lobectomy for stage I non-small cell lung cancer was effective in prevent postoperative complications; while achieving a positive outcome, the Authors do not intend to propose CPAP for all patients who undergo a pulmonary lobectomy, but instead, endorse further research to identify which subgroup of patients could most benefit from this procedure.
Discharging criteria
Discharging criteria included: the cardio-circulatory and respiratory stability, the ability to walk and to do personal grooming with minimal help, an adequate pain control (numerical rating scale <3), absence of intravenous medications requirement, chest tube removed or chest tube with Hemlich valve and minimal air leak. Sporadically, social matters could interfere with the discharge decision. Public holiday and week-end are common discharge days. The discharge is agreed among the chef nurse, the physiotherapist and the consultant in charge.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Ethics committee of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico Milano (No. 2369/2012) and written informed consent was obtained from all patients.
References
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Brunelli A, Cassivi SD, Halgren L. Risk factors for prolonged air leak after pulmonary resection. Thorac Surg Clin 2010;20:359-64. [Crossref] [PubMed]
- Ziarnik E, Grogan EL. Postlobectomy Early Complications. Thorac Surg Clin 2015;25:355-64. [Crossref] [PubMed]
- Yushang C, Zhiyong Z, Xiequn X. The analysis of changes and influencing factors of early postthoracotomy pulmonary function. Chin Med Sci J 2003;18:105-10. [PubMed]
- Jawitz OK, Boffa DJ, Detterbeck FC, et al. Estimating the Annual Incremental Cost of Several Complications Following Pulmonary Lobectomy. Semin Thorac Cardiovasc Surg 2016;28:531-40. [Crossref] [PubMed]
- Imperatori A, Mariscalco G, Riganti G, et al. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J Cardiothorac Surg 2012;7:4. [Crossref] [PubMed]
- Lorut C, Rabbat A, Chatelier G, et al. The place of routine immediate noninvasive ventilation following pulmonary resection in preventing pulmonary complications in patients with COPD (POPVNI Trial). Revue des Maladies Respiratoires 2005;22:127-34. [Crossref] [PubMed]
- Pelosi P, Jaber S. Noninvasive respiratory support in the perioperative period. Curr Opin Anaesthesiol 2010;23:233-8. [Crossref] [PubMed]
- Alexiou S, Panitch HB. Physiology of non-invasive respiratory support. Semin Fetal Neonatal Med 2016;21:174-80. [Crossref] [PubMed]
- Perrin C, Jullien V, Vénissac N, et al. Prophylactic use of noninvasive ventilation in patients undergoing lung resectional surgery. Respir Med 2007;101:1572-8. [Crossref] [PubMed]
- Garutti I, Puente-Maestu L, Laso J, et al. Comparison of gas exchange after lung resection with a Boussignac CPAP or Venturi mask. Br J Anaesth 2014;112:929-35. [Crossref] [PubMed]
- Torres MF, Porfirio GJ, Carvalho AP, et al. Non-invasive positive pressure ventilation for prevention of complications after pulmonary resection in lung cancer patients. Cochrane Database Syst Rev 2015.CD010355. [PubMed]
- Hansen HJ, Petersen RH. Videoassisted thoracoscopic lobectomy using a standardized threeport anterior approach - The Copenhagen experience. Ann Cardiothorac Surg 2012;1:70-6. [PubMed]
- Rivera C, Bernard A, Falcoz PE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg 2011;92:1062-8. [Crossref] [PubMed]
- Park HS, Detterbeck FC, Boffa DJ, et al. Impact of hospital volume of thoracoscopic lobectomy on primary lung cancer outcomes. Ann Thorac Surg 2012;93:372-9. [Crossref] [PubMed]
- Brochard L, Isabey D, Piquet J, et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med 1990;323:1523-30. [Crossref] [PubMed]
- Evans TW. International Consensus Conferences in Intensive Care Medicine: non-invasive positive pressure ventilation in acute respiratory failure. Organised jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Française, and approved by the ATS Board of Directors, December 2000. Intensive Care Med 2001;27:166-78. [Crossref] [PubMed]
- Ferreyra G, Long Y, Ranieri VM. Respiratory complications after major surgery. Curr Opin Crit Care 2009;15:342-8. [Crossref] [PubMed]
- Ireland CJ, Chapman TM, Mathew SF, et al. Continuous positive airway pressure (CPAP) during the postoperative period for prevention of postoperative morbidity and mortality following major abdominal surgery. Cochrane Database Syst Rev 2014.CD008930. [PubMed]
- Aguiló R, Togores B, Pons S, et al. Noninvasive ventilatory support after lung resectional surgery. Chest 1997;112:117-21. [Crossref] [PubMed]
- Ludwig C, Angenendt S, Martins R, et al. Intermittent positive pressure breathing after lung surgery. Asian Cardiovasc Thorac Ann 2011;19:10-3. [Crossref] [PubMed]
- Barbagallo M, Ortu A, Spadini E, et al. Prophylactic use of helmet CPAP after pulmonary lobectomy: a prospective randomized controlled study. Respir Care 2012;57:1418-24. [PubMed]
- Schulz KF, Chalmers I, Grimes DA, et al. Assessing the quality of randomization from reports of controlled trials published in obstetrics and gynecology journals. JAMA 1994;272:125-8. [Crossref] [PubMed]
- Schulz KF. Subverting randomization in controlled trials. JAMA 1995;274:1456-8. [Crossref] [PubMed]
- Altman DG. Compatibility of randomized group. Statistician 1985;34:125-36. [Crossref]