The role of virtual-assisted lung mapping in the resection of ground glass nodules
Introduction
Ground glass nodules (GGNs) are often challenging for thoracic surgeons because of the difficult decision-making process regarding the management of these lesions (1-3). Although the growth of lung adenocarcinoma characterized by GGNs is generally slow or even indolent, some GGNs may grow rapidly during follow-up (4). Accordingly, they are a source of anxiety even for patients with small GGNs. The cost and radiation exposure involved in their long-term monitoring is another cause of concern. A further difficulty is that small GGNs are not easily identified intraoperatively by palpation, particularly in thoracoscopic surgery. Although lung marking techniques have been applied in some patients (5), computer-tomography (CT)-guided needle-mediated methods, the most commonly used marking technique, pose several safety risks, particularly air embolism, which is potentially fatal (6-8). Moreover, conventional marking techniques have inherent limitations. For example, in addition to the intraoperative identification of GGNs, satisfactory resection margins must be acquired but this may be difficult in sublobar lung resection such as wedge resection or segmentectomy. Finally, GGNs frequently present as synchronous multi-centric lesions (9), but conventional single marking techniques may not suffice for their identification.
Virtual assisted lung mapping (VAL-MAP) is a relatively new, preoperative bronchoscopic lung-marking technique that makes use of virtual images, such as obtained by virtual bronchoscopy, to place multiple dye marks, or “tattoos”, on the lung surface (10,11). VAL-MAP is safe, as neither air embolism nor other potentially fatal complications have been reported in patients undergoing the procedure (12). The multiple dye markings of VAL-MAP provide geometric information on the lung surface that aids surgeons not only in identifying a hardly palpable tumor but also in determining oncologically satisfactory resection lines, particularly in patients undergoing thoracoscopic sublobar lung resection (11,13). The multiple markings feature may also be applied to synchronous multi-centric GGNs.
Because of the unique features of VAL-MAP, including its ability to safely mark multiple sites on the lung surface, its introduction can change the clinical strategy for managing GGNs. We recently reported the results of a multi-center prospective study based on 500 patients (12), a number of whom had single or multiple GGNs. The aims of the present study were to retrospectively analyze the data of the previous study, focusing on GGNs, and to reveal the potential role of VAL-MAP in the management of GGNs.
Methods
All patients were prospectively registered in the multi-institutional lung mapping (MIL-MAP) study from 2012 to 2016 (12). Patients included in the study: (I) were scheduled for sublobar lung resection (wedge resection or segmentectomy) to resect a pulmonary lesion and (II) had lesions expected to be hardly palpable intraoperatively or whose resection margins needed to be carefully defined. The study was conducted as a secondary analysis of the MIL-MAP study that was registered as UMIN 000008031 at the University Hospital Medical Information Network Clinical Trial Registry (http://www.umin.ac.jp/ctr/) and conducted under the approval of the ethics committee of each participating hospital. Each patient underwent informed consent regarding the procedure and the prospective clinical study.
A surgical plan was made followed by planning for lung mapping using virtual bronchoscopy together with systems or software as described previously (12). VAL-MAP was conducted 0–3 days before surgery (Figure 1) by either surgeons or respirologists, or both depending on the center’s preference in an endoscopy room equipped with a standard fluoroscope. After the patients were administered local anesthesia and sedated, a bronchoscope was progressed into the target bronchus and 1 mL of blue dye (indigo carmine) was injected through a metal-tip catheter (P6-CW-1, Olympus) toward the visceral pleura under fluoroscopy. After the planned markings had been completed, a CT scan was taken to localize the actual marking on the three-dimensional lung map. The final three-dimensional map was then used to conduct the surgery, usually thoracoscopically.
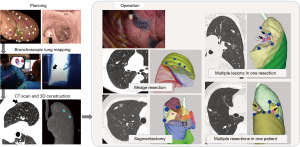
In this study, patients with pure or mixed GGNs were retrospectively selected. Pure GGNs were defined as lung nodules with 100% ground glass opacity, and mixed GGNs as lung nodules containing <100% ground glass opacities. The patient characteristics, lesion characteristics, operation type, and pathological outcome were analyzed. All patients underwent simple segmentectomy, defined as the resection of an anatomical segment(s), complex segmentectomy, defined as single or combined subsegmentectomy, or extended segmentectomy, which extended beyond the anatomical segment into an adjacent segment (14).
The contribution of lung mapping to the success of surgery was then evaluated by the surgeon. Non-parametric tests including Wilcoxon and Kruskal-Wallis tests were used to compare the number of marks among the different types of resection, the diameter and depth of lesions between pure and mixed GGNs. Other statistical analyses were carried out using χ2 tests. The data are expressed as the mean ± SD. The statistical analyses were conducted using JMP version 11.0® (SAS Institute, Cary, NC, USA).
Results
Patients and targeted lesions
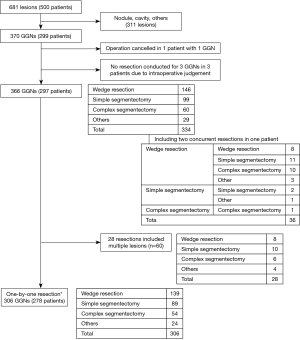
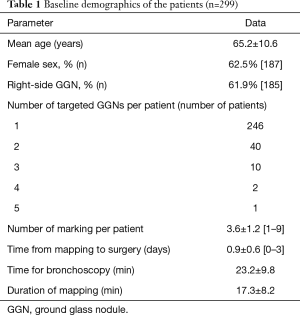
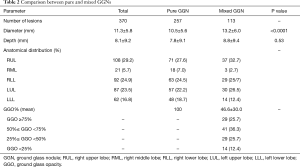
In the original MIL-MAP study, 1,781 markings were made in 500 patients (3.6±1.2 marks/patient) scheduled for resections of 681 pulmonary lesions (1.36 lesion per patient) at 17 centers (12). In this retrospective secondary analysis, 370 GGNs in 299 patients were evaluated (Figure 2). The characteristics of the patients and basic information on their VAL-MAP procedures are shown in Table 1. Right-side GGNs and female sex were dominant. There were 257 pure GGNs and 113 mixed GGNs. Table 2 compares the preoperative characteristics of pure and mixed GGNs. Pure GGNs were significantly smaller than mixed GGNs (10.5±5.6 vs. 13.2±6.0 mm; P<0.0001) and both were most often located in the right upper lobe (~30%). The average percentage of a ground glass component in mixed GGNs was 46.6%±30.0%. The distribution of the ground glass component of mixed GGNs is also shown in Table 2.
Full table
Full table
VAL-MAP and surgery
As shown in Table 1, 1,072 marks were made using VAL-MAP (3.6 marks per patient). The average time between bronchoscope insertion and removal was 23.2 min, and the average duration of the mapping procedure was 17.3 min. One patient developed fever after undergoing VAL-MAP and the subsequent planned operation was canceled. Other minor complications included minor pneumothorax (n=11, 3.7%), pneumomediastinum (n=1, 0.3%), and intrapulmonary bleeding (n=4, 1.3%), none of which affected the following surgery. Therefore, 298 patients underwent operation. Three pure GGNs were not resected based on the intraoperative findings. Resections of other lesions were also scheduled in these three patients, in two of whom, VAL-MAP was conducted for other lesions, too. These three unresected pure GGNs were considered “minor” lesions relative to the major ones (e.g., 5 mm pure GGN vs. 15 mm mixed GGN) and, considering loss of the lung volume and/or distortion of the lungs, surgeons decided not to resect these lesions. Accordingly, 344 resections were eventually intended for 366 GGNs in 297 patients, including 146 wedge resections, 99 simple segmentectomies, 60 complex segmentectomies, 22 wedge resections for biopsy purpose followed by curative segmentectomy (n=3) or lobectomy (n=19), and 7 other resections (Figure 2).
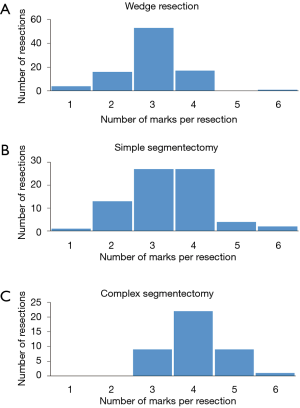
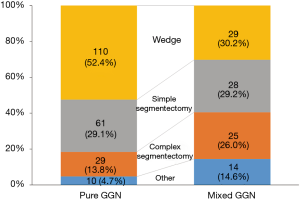
With the exception of the 28 resections that included multiple GGNs in one specimen (in total 61 GGNs), 306 lesions of 278 patients were resected in a one-by-one relationship (Figure 2). In total, 928 marks (3.03 marks per resection) were used for these resections. The largest number of marks were those conducted for complex segmentectomy (4.05±0.74), followed by simple segmentectomy (3.35±0.97) and wedge resection (2.96±0.80) (Figure 3; P=0.001). More than half of the pure GGNs were resected by wedge resection, whereas most of the mixed GGNs were managed by complex segmentectomies (Figure 4; P=0.001).
Multiple GGNs targeted by VAL-MAP
In 53 patients (17.7%), multiple GGNs (225 lesions) were targeted by VAL-MAP for 123 GGNs in total. The strategy in each patient was either one resection to include multiple lesions if they were located close to each other, or multiple resections (Figure 1). Sixty-one GGNs were treated in 28 resections in which multiple lesions were included in once specimen (Figure 2). The resection types were eight wedge resections, ten simple segmentectomies, six complex segmentectomies, and four others. Two concurrent resections were performed in 36 patients (12.1%). The most common combination was wedge resection and simple segmentectomy (11 patients), followed by wedge resection and complex segmentectomy (10 patients), and two wedge resections (8 patients). Resections of multiple lesions in one specimen were relatively common.
Pathological outcomes
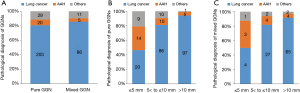
Resection of the targeted lesions was pathologically confirmed for 364 lesions. In addition to the three lesions in which the intraoperative decision was made not to resect them, the resection of two GGNs could not be confirmed by pathology. Thus, the overall rate of successful resection was 98.6%. The pathological outcomes of the successfully resected GGNs are shown in Figure 5. Primary lung cancer was diagnosed in >80% of the resected GGNs, and atypical adenomatous hyperplasia (AAH) in >10%. There was no difference in the distribution of the pathologies between pure and mixed GGNs. However, GGNs >5 mm had a significantly higher ratio of primary lung cancer than those ≤5 mm. AAH was increasingly less frequent and primary lung cancer more frequent with increasing lesion size. This trend was similar between pure and mixed GGNs (P<0.0001; Figure 5B,C).
Contribution of VAL-MAP to surgery
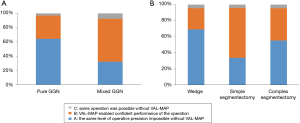
The contribution of VAL-MAP to surgery was evaluated by the surgeons. The contribution was considered high especially in pure GGNs (Figure 6A; P<0.0001). Among the types of lung resection, VAL-MAP contributed the most to wedge resections followed by complex segmentectomy and simple segmentectomy (Figure 6B; P=0.0002).
Discussion
The present study demonstrated that VAL-MAP is a safe and effective preoperative marking technique to identify GGNs for subsequent sublobar resection, including wedge resections and segmentectomy. The multiple markings achieved with VAL-MAP enabled the concurrent resection of multiple small GGNs. The final pathology of the resected lesions revealed a high probability of primary lung cancer for those with a diameter >5 mm. Given the minimal invasiveness of this marking technique and subsequent thoracoscopic sublobar lung resection, the inclusion of VAL-MAP may impact the clinical approach to small GGNs.
The results of the MIL-MAP study revealed the satisfactory safety and effectiveness of VAL-MAP (12). This result was confirmed in the present secondary analysis limited to GGNs. Successful resection was achieved in 98.6% of the cases, and complications were limited. The role of sublobar lung resection in the treatment of early primary lung cancer characterized by GGNs has been described (15,16) and is being further explored (17). VAL-MAP, by facilitating sublobar resection, provides several potential advantages of clinical importance in the field of thoracic surgery. One theoretical advantage of VAL-MAP over conventional lung marking techniques is its ability to achieve multiple markings rather than a single marking (11), which makes it highly resistant to technical errors. Multiple markings serve as a complementary backup in case of a failed marking and thereby increase the probability of successful lung resection (18). In addition, the multiple markings of VAL-MAP provide “geometric information” on the lung surface. Using these multiple reference points on the lung, the surgeon identifies not only the tumor location but also the ideal resection lines yielding sufficient resection margins (11,13,18). This is an important consideration because the acquisition of sufficient resection margins is essential in sublobar resection, as they predict locoregional recurrence and ultimately affect patient survival (19-21). Although reporting of the measured resection margins was not mandatory in our study, the preliminary data indicated the superiority of VAL-MAP over conventional CT-guided percutaneous marking in obtaining resection margins (22).
A further advantage of multiple markings using VAL-MAP is that multiple lesions can be targeted at the same time. Multi-centric synchronous and/or metachronous GGNs in a single patient are a common phenomenon (9). For concurrent multiple small GGNs, surgical resection consisting of multiple concurrent sublobar resections is appropriate (9,23). However, in these cases conventional CT-guided needle-mediated marking is likely to cause pneumothorax, based on a reported rate of 30–50% (5,24). The multiple dye markings made in VAL-MAP allows for multiple concurrent sublobar resections. Indeed, in this study, 36 patients (12.1%) successfully underwent multiple concurrent resections. Although VAL-MAP does not completely avoid the pneumothorax as a potential complication, the incidence in our series was low (~4%). Moreover, the degree of pneumothorax was such that in most affected patients it was barely identifiable in post-mapping CT and thus did not interfere with multiple markings or necessitate additional treatment (12).
The GGNs in this study had a wide range of sizes, and those >5 mm in diameter were found to have a high likelihood of being lung cancer (Figure 5), in agreement with a previous report on the outcomes of subcentimeter GGNs (25). However, whether these relatively small GGNs should be surgically treated is currently a matter of discussion (1-3). Because the intraoperative identification of a tumor is challenging in the absence of an appropriate marking technique, a wait-and-see policy is often justified. The indications for surgery depend on multiple factors, including the danger of the lesion (e.g., invasiveness and growth speed), risk of operation, the patient’s life expectancy, and the patient’s level of anxiety (3). Given the safety and efficacy of VAL-MAP in assisting minimally invasive thoracoscopic sublobar lung resection, the technique may lower the threshold for surgical diagnosis and treatment. Although when a subcentimeter GGNs should be surgically managed remains controversial, the introduction of VAL-MAP may guide decision-making for patients with these lesions.
Although mostly successful, there were cases wherein intended resection was cancelled (n=3) or successful resection was not pathologically confirmed (n=2). The former appears to have happened due to the discrepancy between the surgical plan and actual intraoperative findings, resulting in the surgeons’ judgement to avoid too much resection. Indeed, this is one of the major problems of multi-centric GGNs. All the three lesions have been undergoing careful observation; two of the three remain stable while one has enlarged from 8 to 11 mm but without emergence of a solid component. Regarding the two patients with missed pure GGNs, one has been followed for three years without evidence of any residual disease or local recurrence and the other has undergone completion lobectomy of the right upper lobe, which did not reveal obvious disease. It is probable that the lesions were involved in or very close to the staple line, which made pathological confirmation difficult. Commonly to these two lesions, the location was deep relative to the diameter (diameter and depth were 4 and 16 mm, and 8 and 35 mm, respectively). We recognize that appropriate setting of a resection line for a deeply located lesion is a major limitation of superficial marking techniques including current VAL-MAP (11). As such, the next generation of VAL-MAP is under investigation to overcome the challenge. The original and present study demonstrated an excellent reproducibility among different centers with different settings and personnel. On the other hand, it is true that the process shown in Figure 1 may seem complex and redundant. Thus far, we found all the steps are essential to maintain the quality of VAL-MAP. For example, we found that post-mapping CT is still mandatory because of the possible dislocation of each mark in the present technique of VAL-MAP (18). Electromagnetic navigation bronchoscopy (ENB) already used for lung marking (26,27) may eventually eliminate the necessity of post-mapping CT in VAL-MAP and this is one of the realistic directions VAL-MAP should be further developed toward. In Japan, ENB has been approved recently but only for the biopsy purpose and the cost remains another limitation. We consider it important to prepare a range of options available depending on the availability of different techniques, to accomplish the ultimate goal of satisfactory lung resection.
The present study had several limitations. First, although the original ML-MAP study was prospective, it was single-armed and direct comparison was not made with other marking methods. Although we have compared different techniques previously (11), a fair comparison has been precluded by different indications. Comparison with a historical control has shown a good preliminary result (22) but further investigation is necessary. Second, surgical margins were not evaluated, despite the theoretical advantage of the multiple markings of VAL-MAP in subsequently obtaining sufficient resection margins. In the setting of the original study, a challenge was to standardize the measurement of surgical margins, for example, of deflated versus inflated lungs and the management of staple lines (12). An ongoing study in Japan is prospectively evaluating VAL-MAP in guiding the resection margins of sublobar lung resection. Third, the current multi-center study did not report the details of the decision-making process regarding the indications for surgery or the choice of resection type. For example, some small GGNs were likely to be resected during lobectomy of another lobe, although only lesions whose resections were guided by VAL-MAP were included in this study. Thus, in decision-making regarding the surgical resection of GGNs, the results presented herein should be carefully interpreted.
In conclusion, our study demonstrated the utility of VAL-MAP in the resection of pure and mixed GGNs, including in patients with synchronous multi-centric GGNs. VAL-MAP may allow for less invasive and more accurate thoracoscopic sublobar resection of subcentimeter GGNs in the lung. The use of this new technique may impact decision-making in terms of the timing and type of surgical resection for small GGNs.
Acknowledgements
The authors thank all the participating centers and their staff, represented by Drs. Takuji Fujinaga (Nagara Medical Center, Gifu, Japan), Seijiro Sato, Masanori Tsuchida (Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan), Toshiya Toyazaki, Yasumichi Yamamoto (Shimane Prefectural Central Hospital, Izumo, Japan), Jun-ichi Nitadori, Hideki Kuwano, Masaki Anraku (The University of Tokyo, Tokyo, Japan), Ryo Okabe, Hideaki Miyamoto (Matsue Red Cross Hospital, Matsue, Japan), Fumihiro Tanaka (University of Occupational and Environmental Health, Kitakyushyu, Japan), Junko Tokuno, Cheng-long Huang (Kitano Hospital, Osaka, Japan), Toru Bando, Fumitsugu Kojima (St. Luke’s International Hospital, Tokyo, Japan), Osamu Mishima (Aizawa Hospital, Matsumoto, Japan), Kenji Suzuki (Juntendo University School of Medicine, Tokyo, Japan), Chihiro Takasaki, Yasuhiro Nakashima, Kenichi Okubo (Tokyo Medical and Dental University, Tokyo, Japan), Shin Hirayama (Department of Surgery, Okayama Rosai Hospital, Okayama, Japan), Hiroaki Sakai (Department of Thoracic Surgery, Hygo Prefectural Amagasaki General Medical Center, Amagasaki, Japan), Fumiaki Watanabe (Department of Thoracic Surgery, Matsusaka City Hospital, Matsusaka, Japan), Yasuo Sekine (Department of Thoracic Surgery, Tokyo Women's Medical University Yachiyo Medical Center, Yachiyo, Japan). We thank Dr. Yukari Uemura for her statistical advice. We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. This work was supported by internal funding of the University of Tokyo Hospital, Kyoto University Hospital, and funding from the Japan Agency for Medical Research and Development.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was conducted as a secondary analysis of the MIL-MAP study that was registered as UMIN 000008031 at the University Hospital Medical Information Network Clinical Trial Registry (http://www.umin.ac.jp/ctr/) and conducted under the approval of the ethics committee of each participating hospital. Each patient underwent informed consent regarding the procedure and the prospective clinical study.
References
- Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:108S-30S.
- Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med 2012;185:363-72. [Crossref] [PubMed]
- Zhan P, Xie H, Xu C, et al. Management strategy of solitary pulmonary nodules. J Thorac Dis 2013;5:824-9. [PubMed]
- Lee SW, Leem CS, Kim TJ, et al. The long-term course of ground-glass opacities detected on thin-section computed tomography. Respir Med 2013;107:904-10. [Crossref] [PubMed]
- Sortini D, Feo C, Maravegias K, et al. Intrathoracoscopic localization techniques. Review of literature. Surg Endosc 2006;20:1341-7. [Crossref] [PubMed]
- Kamiyoshihara M, Sakata K, Ishikawa S, et al. Cerebral arterial air embolism following CT-guided lung needle marking. Report of a case. J Cardiovasc Surg (Torino) 2001;42:699-700. [PubMed]
- Sakiyama S, Kondo K, Matsuoka H, et al. Fatal air embolism during computed tomography-guided pulmonary marking with a hook-type marker. J Thorac Cardiovasc Surg 2003;126:1207-9. [Crossref] [PubMed]
- Mizutani E, Nakahara K, Miyanaga S, et al. Kyobu Geka 2012;65:899-902. [Hyperbaric oxygen therapy for air embolism complicating computed tomography (CT)-guided needle marking of the lung]. [PubMed]
- Nakata M, Sawada S, Yamashita M, et al. Surgical treatments for multiple primary adenocarcinoma of the lung. Ann Thorac Surg 2004;78:1194-9. [Crossref] [PubMed]
- Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813-9. [Crossref] [PubMed]
- Sato M. Virtual assisted lung mapping: navigational thoracoscopic lung resection. Cancer Res Front 2016;2:85-104. [Crossref]
- Sato M, Kuwata T, Yamanashi K, et al. Safety and reproducibility of virtual-assisted lung mapping: a multicentre study in Japan. Eur J Cardiothorac Surg 2017;51:861-8. [PubMed]
- Sato M, Murayama T, Nakajima J. Techniques of stapler-based navigational thoracoscopic segmentectomy using virtual assisted lung mapping (VAL-MAP). J Thorac Dis 2016;8:S716-30. [Crossref] [PubMed]
- Tsubota N, Ayabe K, Doi O, et al. Ongoing prospective study of segmentectomy for small lung tumors. Study Group of Extended Segmentectomy for Small Lung Tumor. Ann Thorac Surg 1998;66:1787-90. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. [Crossref] [PubMed]
- Aokage K, Saji H, Suzuki K, et al. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg 2017;65:267-72. [Crossref] [PubMed]
- Sato M, Nagayama K, Kuwano H, et al. Role of post-mapping computed tomography in virtual-assisted lung mapping. Asian Cardiovasc Thorac Ann 2017;25:123-30. [Crossref] [PubMed]
- Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. [Crossref] [PubMed]
- El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol 2007;14:2400-5. [Crossref] [PubMed]
- Wolf AS, Swanson SJ, Yip R, et al. The Impact of Margins on Outcomes After Wedge Resection for Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;104:1171-8. [Crossref] [PubMed]
- Kawashima S, Nagayama K, Sato M, et al. Comparative review of sublobar resection before and after virtual assisted lung mapping introduction in a single institution. Interact Cardiovasc Thorac Surg 2016;23:i21-i. [Crossref]
- Mun M, Kohno T. Single-stage surgical treatment of synchronous bilateral multiple lung cancers. Ann Thorac Surg 2007;83:1146-51. [Crossref] [PubMed]
- Dendo S, Kanazawa S, Ando A, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology 2002;225:511-8. [Crossref] [PubMed]
- Shao G, Ren W, Feng Z, et al. The role of video-assisted thoracoscopic surgery in management of the multiple ground-glass nodules. Indian J Cancer 2015;52 Suppl 2:e75-9. [Crossref] [PubMed]
- Zhao ZR, Lau RW, Ng CS. Hybrid theatre and alternative localization techniques in conventional and single-port video-assisted thoracoscopic surgery. J Thorac Dis 2016;8:S319-27. [PubMed]
- Awais O, Reidy MR, Mehta K, et al. Electromagnetic Navigation Bronchoscopy-Guided Dye Marking for Thoracoscopic Resection of Pulmonary Nodules. Ann Thorac Surg 2016;102:223-9. [Crossref] [PubMed]


