Effect of lung protective ventilation on coronary heart disease patients undergoing lung cancer resection
Introduction
The mechanical ventilation, especially the large tidal volume (Vt) one-lung ventilation (OLV), can cause ventilator-induced lung injury (VILI) in clinical surgery (1-3). In the VILI, cytokines play an important role in the inflammatory response in lung. Cytokines produced locally in the lung from VILI enter peripheral blood to recruit inflammatory cells back to the lung. The cytokines of peripheral blood can subsequently aggravate the lung injury (4-6). Meanwhile, Cytokine bridging between systemic inflammation and local inflammatory reactions has been identified in local coronary atherosclerotic plaques, which regulates the local immune response and influences the stability of plaques, and therefore it is the important factor influencing the prognosis of coronary heart disease (CHD) patients (7-9).
In China, CHD and lung cancer are the two leading causes of death (10,11). It is reported that about 10% of lung cancer patients are accompanied by CHD (12). Lung resection surgery employing OLV was most often used to treat lung cancer in China. However, in patients with both lung cancer and CHD, poor cardiac function, weak exercise tolerance, lung lesions and impaired lung function increase their risk for VILI during surgery (13,14). In turn, because cytokine release during VILI adversely affects atherosclerotic plaques during lung cancer resection, minimization of pulmonary inflammatory responses by reduction of cytokine levels is recommended during the anesthesia period of lung cancer resection with OLV. Schultz et al. (15) reported that low Vt and positive end-expiratory pressures (PEEP) are the main components of lung-protective ventilation (LPV). But use of very low levels of PEEP could lead to atelectasis with ventilation strategies that incorporate lower Vt. High PEEP could cause lung overdistension and circulatory compromise (16). Previous studies have demonstrated that LPV with small Vt and appropriate PEEP reduced lung inflammation (17,18). However, it is not clear whether the reduction of inflammatory cytokines can effectively prevent the lung injury during the lung cancer resection OLV period of CHD patients. In this study, the CHD patients treated with lung cancer resection were taken as the subjects to explore the effect of LPV on the perioperative inflammatory response in CHD patients undergoing the video-assisted thoracoscopic lung cancer resection.
Methods
Patient selection and assignment to study groups
This is a single center, randomized controlled trial. Primary endpoint of the study are plasma concentrations of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-10 and C-reactive protein (CRP). Secondary endpoints include respiratory variables and hemodynamic variables. The study has been registered in the Chinese Clinical Trial Registry (ChiCTR-OOC-16009112). The screening started in September 2016, and a recruitment time of 11 months has been defined based on the incidence of lung surgery and patient characteristics at our hospital. Written informed consent was obtained from all participants prior to surgery. In order to standardize research process and ensure the test quality, we developed flow diagram (as shown Figure 1) through referring some literature and consulting experts, combined with the actual situation of our hospital.
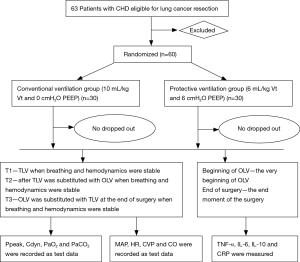
Patients, including 32 males and 28 females with American Society Anesthesiologists’ physical status II–III were chosen for study. Patient ages ranged between 40 and 65 years and patients who were scheduled for video-assisted thoracoscopic lung cancer resection were recruited. All patients were classified into either a volume-controlled ventilation (VCV) group (C group) or protective ventilation (PV) group (P group) according to a controlled, randomized design produced by computer-generated codes. Each group contained 30 patients. C group: Vt =10 mL/kg, PEEP =0 cmH2O; P group: Vt =6 mL/kg, PEEP =6 cmH2O.
Patients scheduled for thoracic surgery under OLV general anesthesia were eligible if they either (1) had proven CHD or (2) had ≥2 risk factors for CHD. Proof of CHD was predefined as a history of myocardial infarction or coronary revascularization, ≥50% stenosis on coronary angiography or myocardial ischemia induced either by radionuclide or echocardiographic stress testing. Predefined risk factors for CHD were diabetes mellitus requiring treatment (oral antidiabetic medications or insulin), arterial hypertension, history of stroke, functional capacity of <4 metabolic equivalent tasks or abnormal ECG (signs of left ventricular hypertrophy, left bundle branch block or abnormalities of the ST-segment or T-wave).
The exclusion criteria were as follows: (I) current congestive heart failure; (II) current unstable angina pectoris; (III) preoperative hemodynamic instability; (IV) hepatic disease; (V) severe chronic obstructive pulmonary disease; (VI) renal insufficiency; (VII) emergent surgery; (VIII) pregnancy; (IX) concurrent enrollment in another RCT; (X) absence of written informed consent or other factors, including long-term use of antibiotics and immunosuppressive agents, chest and abdomen deformity, and body mass index greater than 24.
Presurgical preparation, anesthesia and ventilation
Angina was effectively relieved before surgery. Patients were brought into the operation room without premedication and preoxygenation was ensured by delivery of 100% oxygen through a facial mask. After a venous line was inserted, 0.03 mg/kg midazolam was administered. Standard monitoring procedures involving ECG, SpO2 and bispectral index (BIS, Philips Healthcare, Andover, MA, USA) were performed. A 20G arterial catheter was inserted percutaneously into the radial artery of the arm on the contralateral side relative to the surgical site under local anesthesia for continuous blood pressure monitoring and collection of blood samples. When patient mean arterial pressure (MAP) and heart rate (HR) were stable, the measured MAP and HR values served as base values. General anesthesia was induced with 0.3 mg/kg etomidate and 0.5 µg/kg sufentanil. After loss of consciousness, 1.2 mg/kg rocuronium was administered for intubation and manual facemask ventilation was continued until muscle relaxation was achieved. The BIS was maintained between 40 and 50 to allow tracheal intubation. MAP and HR were prevented from exceeding 20% of baseline values using vasoactive drugs. All patients were intubated with a left double-lumen endobronchial tube (DLT, Mallinckrodt-Endobronchial Tube, Covidien, Made in Ireland. no. 37 for male and no. 35 for female patients) and the proper position of the DLT was confirmed using fiberoptic bronchoscopy. After anesthesia induction and intubation, all patients in both groups were ventilated using a GE Datex Ohmeda S/5 Avance Anesthesia Machine (S/5; Datex Ohmeda, Helsinki, Finland). Initially, two-lung ventilation (TLV) with VCV was performed using 1.0 fraction of inspired oxygen concentration (FiO2), a Vt of 8 mL/kg of ideal body weight, 12 breaths/min respiration rate, an inspiration-to-expiration ratio (I:E) of 1:1.5 and no inspiratory time pause in both groups. The respiration rate was adjusted to maintain an End-tidal carbon dioxide concentration (ETCO2) of 35–45 mmHg. An indwelling bladder catheter was inserted and a three-lumen central venous catheter was inserted from the right internal jugular vein for monitoring of central venous pressure (CVP) and infusion. Maintenance of anesthesia included a continuous infusion of propofol (5 mg/kg/h), sufentanil (0.4 µg/kg/h) and atracurium (0.15 mg/kg/h) that was followed 30 min after intubation. After changing the patient to a lateral decubitus position, the location of the DLT was reassessed using fiberoptic bronchoscopy.
Lung resection procedure
At the start of creation of the incision, OLV was subsequently performed with C group or P group according to the group allocation. Patients in the C group were ventilated with Vt 10 mL/kg and 0 cmH2O PEEP, while those in the P group were ventilated with Vt 6 mL/kg and 6 cmH2O PEEP. The inspiration-to-expiration ratio (I: E) was 1:1.5 and FIO2 was 100% and no inspiratory time pause was set in two groups. ETCO2 was maintained at 35–40 mmHg during OLV by adjusting the respiratory rate. OLV was changed to TLV until incision closure. TLV modes used were the same as used previously. The depth of anesthesia was maintained similarly between BIS 40 and 60 in both groups. At the end of the operations, all patients were transferred to the ICU. Surgeries of all patients were performed by the same team of thoracic surgeons and were managed by the same team of anesthesiologists. During surgery, SpO2 was maintained above 95% at all times. If SpO2 fell below 95%, the following treatments were performed: aspiration of sputum, checking and adjustment of the catheter position by fiberoptic bronchoscopy, lung recruitment maneuver, change of Vt, continuous positive airway pressure or TLV. If SpO2 could not be restored to greater than 90%, the patient would be removed from the study. 6% HES 130/0.4 (Voluven®; Fresenius Kabi, Germany) and Ringer’s solution were used to maintain stable arterial pressure and HR. If variations in MAP and HR exceeded 20% of baseline values for longer than 5 minutes, 5 mg of ephedrine or 0.5 mg atropine was administered intravenously. The nasopharyngeal temperature was monitored and body temperature was maintained between 36 to 38 °C. No patients were dropped from this study.
Patient monitoring during surgery
The peak inspiratory pressure (Ppeak), dynamic compliance (Cdyn), MAP, HR, CVP and cardiac output (CO) were continuously measured. Ppeak, Pmean, Cdyn, MAP, HR, CVP and CO were recorded as test data at three-time points: T1—endotracheal intubation for TLV when breathing and hemodynamics were stable; T2—after TLV was substituted with OLV when breathing and hemodynamics were stable; T3—OLV was substituted with TLV at the end of surgery when breathing and hemodynamics were stable. At T1, T2 and T3, arterial blood was collected for blood gas analysis of arterial oxygen tension (PaO2) and arterial carbon dioxide tension (PaCO2).
Sample collection for cytokine measurement
A volume of arterial blood of 3 ml was collected at the very beginning of OLV (beginning of OLV) and the end moment of the surgery (end of surgery) and added into sodium citrate anticoagulant tubes, followed by centrifugation at 3,000 r/min for 10 minutes. Supernatants were collected and preserved at −70 °C for testing. The concentrations of TNF-α, IL-6, IL-10 and CRP in patient blood in both groups at the very beginning of OLV and the end moment of the surgery were measured using ELISA. At the same time the arterial blood sample was drawn, blood gases were analyzed bedside using an I-STAT bedside blood-gas analyzer (USA).
Sample size calculations
The sample size estimation was performed on the basis of IL-6. A pilot study involving 6 patients (n1=n2=3) at our medical center found the mean ± standard deviation of IL-6 to be 109.994±20.253 and 151.205±30.397. A sample size of 56 patients was required to observe a significant reduction of 20% in level of IL-6 at a power of 95% and two-sided significance level of 0.05. To compensate for the possibility of dropout, we recruited 60 patients (30 patients per group).
Statistical analysis
All data were statistically analyzed using SPSS for Windows, version 17.0 (SPSS, Chicago, IL, USA). The data were statistically prepared as the mean ± standard deviation. Depending on the distribution of the data, a paired t-test of Wilcoxon signed rank test was used within the same group. Intergroup comparison of results at a given study time point was carried out using the independent sample t-test. Values of P<0.05 were considered statistically significant.
Results
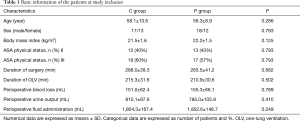
There were no significant differences in demographic data, surgical characteristics, or intraoperative variables between the two groups (P>0.05) (Table 1).
Full table
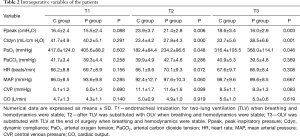
At three points time hemodynamic variables, such as HR, MAP, CVP and CO, did not differ between two groups (respectively P=0.308, 0.667, 0.083, 0.619). Ppeak in P group was significantly lower than that in C group at T2 and T3 (respectively P=0.006, 0.003). However, there were significant differences in Cldyn between these two groups at T2 and T3 (respectively P=0.000, 0.001). PaO2 in P group was also significantly higher than that in C group at T2 and T3 (respectively P=0.048, 0.046). As to PaCO2, we did not find any difference between these two groups at three-time point (respectively P=0.258, 0.286, 0.369). And they were within normal limits in both groups (Table 2).
Full table
There were no significant differences in plasma concentrations of TNF-α、IL-6, and CRP between the two groups at the very beginning of OLV (C group TNF-α P=0.532; IL-6 P=0.101; IL-10 P=0.076; CRP P=0.202); however, the concentrations of TNF-α, IL-6, IL-10, CRP at the very beginning of OLV were significantly lower than that at the end moment of the surgery in both groups (C group TNF-α P=0.00006, IL-6 P=0.0002, IL-10 P=0.000007, CRP P=0.00001; P group TNF-α P=0.02,IL-6 P=0.0063,IL-10 P=0.00002,CRP P=0.042). At the end moment of the surgery, although the P group tended to exhibit higher TNF-α and IL-10 values than the C group, the differences did not reach statistical significance (TNF-α P=0.0817,IL-10 P=0.0635). In contrast, at the end moment of the surgery, compared with C group, IL-6 and CRP were lower in P group, the differences were statistically significant (IL-6 P=0.0093, CRP P=0.0005) (Table 3).

Full table
Discussion
Cytokines are key substances that mediate nonspecific inflammatory responses throughout the body. Their over-expression can cause local or systemic pathological damage and can be triggered by surgical procedures that damage tissues, such as lung cancer resection surgery and mechanical ventilation. Tissue damage from mechanical ventilation during lung cancer resection, especially from the use of large Vt OLV, can cause cytokine-mediated lung inflammation, known as VILI (1,2,3,19,20). Meanwhile, inflammation has been demonstrated to be associated with occurrence and progression of CHD (21). This association has been strengthened by studies demonstrating increased cytokine levels within coronary atherosclerotic plaques as evidence that cytokines influence the plaque stability and regulate bridge systemic and local inflammatory reactions (22,23). Within localized coronary atherosclerotic plaques, cytokines such as TNF-α,IL-6 and IL-10 are important regulatory inflammatory factors. Consequently, they play a vital role in the formation and evolution of CHD (24). Moreover, CRP, an acute phase response protein, is generated by the liver in response to inflammatory stimulation. CRP has served as a peripheral marker for monitoring inflammation that can play a direct role in coronary atherosclerotic plaque rupture (25,26). Thus, TNF-α, IL-6, IL-10 and CRP are considered important players in CHD occurrence and development and are predictive of ultimate CHD prognosis.
The mechanical ventilation is a double-edged sword. It is a commonly used treatment methodology in clinical anesthesia, but it can also cause VILI. In routine clinical practice, a large Vt is routinely used in order to maintain adequate oxygenation. However, a number of recent studies have demonstrated that large Vt and high airway pressure are main risk factors underlying pulmonary inflammation, especially for OLV patients (1-3,27,28). In addition, lateral positioning during lung resection combined with a relatively large Vt would induce excessive airway pressure causing excessive alveolar expansion. Unfortunately, such large Vt levels caused alveolar volume injury, pressure injury, shear injury, pulmonary inflammatory cell activation and release of numerous cytokines into the blood. Meanwhile, peripheral blood cytokines have been actually shown to exacerbate the initial lung injury. Meanwhile, cardiac ischemia in patients with CHD causes abnormal segmental wall movement, poor activity tolerance, presence of pulmonary lesions and impaired pulmonary function cause the lung to be more susceptible to inflammation during OLV (13,14). LPV has recently been developed to reduce VILI and simultaneously improve systemic oxygenation. LPV regulates PEEP, maintains a greater number of pulmonary alveoli in an open state, avoids elevation of end-expiratory lung volume, helps maintain target Vt, and alleviates injuries caused by elevated lung volume and abnormal Vt. PEEP can prevent the collapse of open pulmonary alveoli, maintain lung volume and function of pulmonary surfactants, and reduce the shear stress caused by repeated/loss of alveolar (5,15,16,18). So LPV was used for CHD patients undergoing lung resection during OLV in this study.
TNF-α, the initiating factor of an inflammatory response, is the most important proinflammatory factor present early during inflammation. TNF-α acts to further induce generation of IL-6 and IL-10 during activation of the inflammatory cascade response, whereby IL-10 inhibits up-regulation of expression of TNF-α and IL-6 (4,23,29,30).
Compared with the very beginning of OLV, TNF-α levels in patients of both P and C groups all increased at the end moment of the surgery. However, the rate of TNF-α increase in the P group was significantly lower than in the C group, although the difference was not statistically significant. Plausible reasons for the lack of a large intergroup difference include the presence of numerous stimuli triggering TNF-α production in this study, earlier TNF-α generation during the procedure or the possibility that mechanical ventilation was not the main factor underlying observed TNF-α increases. Thus, we observed an increase of TNF-α concentrations in both groups at the end moment of the surgery. However, the TNF-α concentration of the P group was lower overall due to effects unique to LPV, although this intergroup difference was not statistically significant.
Previous studies have demonstrated that mechanical ventilation, surgical trauma and stress lead to a sharp increase of pro-inflammatory cytokine IL-6. Moreover, IL-6 is considered an indicator of the severity of tissue injury and trauma. During elective surgery, IL-6 levels often rise 2 h after making a skin incision. In addition, IL-6 has been observed to stimulate production of CRP, another indicator of inflammation, within a few hours after the start of acute inflammation and tissue damage in the body (31,32). Meanwhile, IL-10 is an important anti-inflammatory factor that plays a protective role in the systemic inflammatory response. As observed for IL-6, it has been reported that a large quantity of IL-10 is generated about 4h after making an incision (4,23,29,30). Therefore, in both groups we observed an IL-10 level increase at the end moment of the surgery. A lower increase was observed in the P group than the C group, but the difference between groups was not statistically significant. We hypothesized that the IL-10 level was lower in the LPV-treated P group because the IL-6 level in that group was lower. However, due to the short duration of surgery, IL-10 production did not reach peak levels. Consequently, a significant difference in IL-10 level between groups was not observed.
The results of this study show that, compared with the very beginning of OLV, the concentrations of IL-6 and CRP in patients of the two groups all increased at the end moment of the surgery, but the increase rate of the P group was significantly lower than that of the C group. Moreover, at both time points, the Ppeak of P group was significantly reduced relative to that of the C group, PaO2 and Cdyn improved. These results may be due to VILI, surgical trauma, etc., resulting from the generation of cytokines in lung and their release into the peripheral blood. Once in the blood, the cytokines would further stimulate the liver to produce CRP. Because the P group received LPV, these patients exhibited less cytokines released from lung in the peripheral blood with improved lung function as compared to the C group patients. Furthermore, LPV may also reduce alveolar ventilation irritation and lung inflammatory cytokine responses by reduction of ventilation pressure for increased lung compliance, etc. Moreover, LPV exerted no negative effects on patient hemodynamics. For these reasons, we hypothesized that LPV could effectively reduce peripheral blood inflammatory factor levels in CHD lung resection patients treated with OLV. Therefore, LPV should improve lung ventilation function and clinical safety by triggering fewer adverse effects on coronary atherosclerotic plaque.
The possible reason why the peripheral blood TNF-α and IL-6 concentrations in this study are higher than that in Serpa et al. (33) study is that the research object are different. Serpa Neto chose the patients with laparotomy, and we chose the lung cancer patients with CHD. There is an inflammatory reaction in coronary atherosclerosis, cytokines play a vital role in the formation and evolution of CHD. The lung of patients with lung cancer is accompanied by inflammatory reaction. So there are more cytokines in the patients. The possible reasons why postoperative peripheral blood TNF-α, IL-6 concentrations are higher are listed below. When performed the lung cancer resection surgery, the lung underwent external injury from both the operation and mechanical one lung ventilation. When the resection surgery finished, the lung underwent the ischemia-reperfusion, which aggravated the lung injury. All the injuries stimulated the cytokines production in the lung, and the cytokines were more likely released into the peripheral blood. We hypothesized that when lung cancer patients with CHD underwent lung resection surgery, lung tissue acted as the cytokines storage devices, once the lung got further injured, more cytokines were stimulated to release into peripheral blood. Our future research will focus on how to reduce the inflammatory reaction. In addition, anesthetic factors may also be the reason why the TNF-α, IL-6 levels are different. The total intravenous percentage in our study is 100%, while in Serpa Neto 's study, the percentage is only 12.7–13.7%, the left are Mixed (volatile and intravenous) anesthesia, the volatile has certain inhibitory effect on cytokines production.
Because previous studies have not agreed upon one best Vt and PEEP level for OLV, most scholars have subscribed to the principle of individualized synthetic therapy (34,35). We therefore tested the available information in relevant references by conducting pilot experiments and initially set the Vt of the P group to 6 mL/kg and the PEEP to 6 cmH2O. However, although PEEP could effectively prevent alveolar collapse and atelectasis (36), we realized that excessive PEEP would cause increased inspiratory pressure, reduced lung compliance and impaired lung function (37,38). Due to the fact that increased pressure on the chest would adversely affect hemodynamics (39), PEEP was ultimately set to 6 cmH2O, even though small Vt surgery might cause atelectasis, premature closure of alveoli and other hypoventilation circumstances.
In particular, most CHD patients are elderly and exhibit poor cardiopulmonary function. Moreover, these patients are often afflicted with lung diseases that result in lung collapse upon lateral positioning during chest surgery (14). Therefore, insufficient oxygen supply and CO2 accumulation were valid concerns in this study. Consequently, low-level PEEP was used in patients of the P group, while all patients received intermittent hyperinflation, removal of respiratory tract secretions and other interventions to avoid atelectasis and also avoid sustained low SpO2 and sustained high PETCO2. Cai. reported, using CT scans of total lung volume, that small Vt ventilation did not increase the incidence of atelectasis (40). Meanwhile, several other researchers demonstrated that PEEP could effectively prevent alveolar collapse and atelectasis and maintain end-expiratory alveoli in a relatively open state (17,36,41). In our study no chest X-rays or other kinds of imaging suitable for detecting atelectasis were performed, so we can not evaluate atelectasis, but we have observed enough oxygenation that is very helpful to CHD as demonstrated in this study.
Here we should mention that steps were taken to minimize errors resulting from variability in implementation. First, although we considered studying the effects of inhaled anesthetics on inflammatory factors (29), inhaled anesthetics were not used. In addition, all surgical procedures were performed by the same group of surgeons, which minimized variations in outcomes from differences in surgical technique. Moreover, same depth of anesthesia was employed during surgeries. Finally, differences in operation duration, mechanical ventilation time, blood loss, urine output and fluid administration were not statistically significant and we minimized the effect of other factors on the cytokine assay results.
With the exception of mechanical ventilation used in acute respiratory distress syndrome ICU patients, general anesthesia administered in the operating room usually requires a shorter mechanical ventilation time that results in only mild or no lung injury (42,43). Moreover, disease in the vast majority of patients is not complicated by lung disease, raising questions as to whether implementation of LPV can benefit most patients. In recent years, the results of clinical studies have also been controversial. It has been reported that LPV was not beneficial to healthy lungs, while the use of PEEP may actually increase likelihood of lung injury (44,45), this may be because larger PEEP leads to greater airway pressure and formation of pulmonary edema. Conversely, Wrigge et al. (42) found that mechanical ventilation exerted no significant effects on lung or on systemic inflammatory cells in patients undergoing surgery of duration of less than 3 hours. The possible reasons for these contrasting results are that the patients in Wrigge’s experiments were subject to non-pulmonary surgery, possessed good lung function or that the mechanical ventilation time used was too short for cytokines to be fully produced and released.
Other studies have shown that failure to use PEEP may result in worse clinical outcomes, including hypoxemia, pneumonia from mechanical ventilation and other complications (46,47). This may be due to the fact that poor lung function of some patients is frequently complicated by atelectasis. For these patients, the use of PEEP can improve lung compliance and oxygenation. Although the researchers in this study failed to reach an agreement on whether LPV can reduce the inflammatory response, they did agree that LPV could increase oxygenation and improve respiratory function.
VILI poses many risks during thoracic surgery of which OLV is one of the important risk factors (4-6). However, OLV is essential in pulmonary lobectomy. Consistent with other studies (4,6,29,48), our findings have confirmed that the use of LPV in OLV can decrease the production of inflammatory factors and improve oxygenation. Nowadays, most studies have shown that conventional ventilation of high Vt used during chest surgery is detrimental, while OLV application using small Vt combined with a moderate PEEP level can improve patient prognosis (5,28,49). However, these results await confirmation by additional independent randomized controlled trials.
However, in this work we observed the inflammatory response only through measurement of inflammatory factors in peripheral blood. Moreover, we did not continuously monitor postoperative pulmonary and systemic inflammatory responses or monitor cytokines within alveolar lavage fluid. In addition, the results reported here reflect a short observation time for evaluation of the inflammatory response and the study lacked a comprehensive scope. Thus, further studies are still needed to evaluate short-term and long-term effects of LPV on coronary atherosclerotic plaques and to develop ways to optimize LPV and implement individualized LPV. This study was only one clinical study using a small sample size performed in a single center. Thus, additional clinical studies are needed to independently verify the results presented here.
In summary, LPV can effectively reduce the OLV airway pressure of CHD patients during pulmonary resection, improve Cdyn, PaO2 and reduce concentrations of IL-6 and CRP to subsequently alleviate the perioperative inflammatory response.
Acknowledgements
Funding: This work was supported by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201810); and Beijing Health System High-level Health Technical Talents Cultivation Fund [2013-2-004]; and Young Scholar Research Grant of Chinese Anesthesiologist Association (220160900010).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was conducted in accordance with the Helsinki declaration and with approval from the Ethics Committee of Beijing Anzhen Hospital (NO. 2016006X) and was approved by the Clinical Research Ethics Committees of our hospital and was registered in the Chinese Clinical Trial Registry (ChiCTR; registration number ChiCTR-OOC-16009112). Written informed consent was obtained from all participants.
References
- Kozian A, Schilling T, Röcken C, et al. Increased alveolar damage after mechanical ventilation in a porcine model of thoracic surgery. J Cardiothorac Vasc Anesth 2010;24:617-23. [Crossref] [PubMed]
- Jeon K, Yoon JW, Suh GY, et al. Risk factors for post-pneumonectomy acute lung injury/acute respiratory distress syndrome in primary lung cancer patients. Anaesth Intensive Care 2009;37:14-9. [PubMed]
- Shen Y, Zhong M, Wu W, et al. The impact of tidal volume on pulmonary complications following minimally invasive esophagectomy: a randomized and controlled study. J Thorac Cardiovasc Surg 2013;146:1267-73; discussion 1273-4. [Crossref] [PubMed]
- Gama de Abreu M, Heintz M, Heller A, et al. One-lung ventilation with high tidal volumes and zero positive end-expiratory pressure is injurious in the isolated rabbit lung model. Anesth Analg 2003;96:220-8. table of contents. [Crossref] [PubMed]
- Bender SP, Paganelli WC, Gerety LP, et al. Intraoperative lung-protective ventilation trends and practice patterns: a report from the multicenter perioperative outcomes group. Anesth Analg 2015;121:1231-9. [Crossref] [PubMed]
- Della Rocca G, Coccia C. Acute lung injury in thoracic surgery. Curr Opin Anaesthesiol 2013;26:40-6. [Crossref] [PubMed]
- Bhagat K, Vallance P. Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation 1997;96:3042-7. [Crossref] [PubMed]
- Hojo Y, Shimada K. Role of cytokines in acute coronary syndrome. Nihon Rinsho 1998;56:2500-3. [PubMed]
- Geng YJ, Wu Q, Muszynski M, et al. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-γ, Tumor necrosis factor–α, and interleukin-1β. Arterioscler Thromb Vasc Biol 1996;16:19-27. [Crossref] [PubMed]
- Jiang G, Wang D, Li W, et al. Coronary heart disease mortality in China: age, gender, and urban-rural gaps during epidemiological transition. Rev Panam Salud Publica 2012;31:317-24. [Crossref] [PubMed]
- Chen PC, Muo CH, Lee YT, et al. Lung cancer and incidence of stroke: a population-based cohort study. Stroke 2011;42:3034-9. [Crossref] [PubMed]
- Ma X, Huang F, Zhang Z, et al. Lung cancer resection with concurrent off-pump coronary artery bypasses: safety and efficiency. J Thorac Dis 2016;8:2038-45. [Crossref] [PubMed]
- Soriano JB, Rigo F, Guerrero D, et al. High prevalence of undiagnosed airflow limitation in patients with cardiovascular disease. Chest 2010;137:333-40. [Crossref] [PubMed]
- Minasian AG, Elshout FJVD, Dekhuijzen PR, et al. Serial pulmonary function tests to diagnose COPD in chronic heart failure. Transl Respir Med 2014;2:12. [Crossref] [PubMed]
- Schultz MJ, Serpaneto A. Optimizing perioperative mechanical ventilation as a key quality improvement target. Rev Bras Ter Intensiva 2015;27:102-4. [Crossref] [PubMed]
- PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology, Hemmes SN, Gama de Abreu M, et al. High versus low positive end-expiratory pressure during general anesthesia for open abdominal surgery (PROVHILO trial): a multicenter randomised controlled trial The PROVE. Lancet 2014;384:495-503. [Crossref] [PubMed]
- Bitker L, Richard JC. Intensive alveolar recruitment strategy in the post-cardiac surgery setting: one PEEP level may not fit all. J Thorac Dis 2017;9:2288-92. [Crossref] [PubMed]
- Hoegl S, Zwissler B. Preventing ventilator-induced lung injury-what does the evidence say? J Thorac Dis 2017;9:2259-63. [Crossref] [PubMed]
- Berngard SC, Beitler JR, Malhotra A. Personalizing mechanical ventilation for acute respiratory distress syndrome. J Thorac Dis 2016;8:E172-4. [Crossref] [PubMed]
- Slinger P. Pro: low tidal volume is indicated during one-lung ventilation. Anesth Analg 2006;103:268-70. [Crossref] [PubMed]
- Golia E, Limongelli G, Natale F, et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep 2014;16:435. [Crossref] [PubMed]
- Kishimoto T, Akira S, Narazaki M, et al. Interleukin-6 family of cytokines and gp130. Blood 1995;86:1243-54. [PubMed]
- Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev 2006;86:515-81. [Crossref] [PubMed]
- Schieffer B, Schieffer E, Hilfikerkleiner D, et al. Expression of Angiotensin II and Interleukin 6 in Human Coronary Atherosclerotic Plaques Potential Implications for Inflammation and Plaque Instability. Circulation 2000;101:1372-8. [Crossref] [PubMed]
- Goei D, Hoeks SE, Boersma E, et al. Incremental value of high-sensitivity C-reactive protein and N-terminal pro-B-type natriuretic peptide for the prediction of postoperative cardiac events in noncardiac vascular surgery patients. Coron Artery Dis 2009;20:219-24. [Crossref] [PubMed]
- Pearson TA, Mensah GA, Hong Y, et al. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease Application to Clinical and Public Health Practice: Overview. Circulation 2004;110:e543-4. [Crossref] [PubMed]
- Wrigge H, Pelosi P. Tidal volume in patients with normal lungs during general anesthesia: lower the better? Anesthesiology 2011;114:1011-3. [Crossref] [PubMed]
- Brassard CL, Lohser J, Donati F, et al. Step-by-step clinical management of one-lung ventilation: continuing professional development. Can J Anaesth 2014;61:1103-21. [Crossref] [PubMed]
- Michelet P, D'Journo XB, Roch A, et al. Protective ventilation influences systemic inflammation after esophagectomy: a randomized controlled study. Anesthesiology 2006;105:911-9. [Crossref] [PubMed]
- Wilson MR, Choudhury S, Takata M. Pulmonary inflammation induced by high-stretch ventilation is mediated by tumor necrosis factor signaling in mice. Am J Physiol Lung Cell Mol Physiol 2005;288:L599-607. [Crossref] [PubMed]
- McBride WT, Armstrong MA, Crockard AD, et al. Cytokine balance and immunosuppressive changes at cardiac surgery: contrasting response between patients and isolated CPB circuits. Br J Anaesth 1995;75:724-33. [Crossref] [PubMed]
- Butler J, Rocker GM, Westaby S. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 1993;55:552-9. [Crossref] [PubMed]
- Serpa Neto A, Campos PP, Hemmes SN, et al. Kinetics of plasma biomarkers of inflammation and lung injury in surgical patients with or without postoperative pulmonary complications. Eur J Anaesthesiol 2017;34:229-38. [Crossref] [PubMed]
- Suzumura EA, Figueiró M, Normilio-Silva K, et al. Effects of alveolar recruitment maneuvers on clinical outcomes in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Intensive Care Med 2014;40:1227-40. [Crossref] [PubMed]
- Ladha K, Melo MFV, Mclean DJ, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ 2015;351:h3646. [Crossref] [PubMed]
- Sarge T, Loring SH, Yitsak-Sade M, et al. Raising positive end-expiratory pressures in ARDS to achieve a positive transpulmonary pressure does not cause hemodynamic compromise. Intensive Care Med 2014;40:126-8. [Crossref] [PubMed]
- Retamal J, Borges JB, Bruhn A, et al. High respiratory rate is associated with early reduction of lung edema clearance in an experimental model of ARDS. Acta Anaesthesiol Scand 2016;60:79-92. [Crossref] [PubMed]
- Neto AS, Hemmes SNT, Barbas CSV, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med 2016;4:272-80. [Crossref] [PubMed]
- Coppola S, Froio S, Chiumello D. Protective lung ventilation during general anesthesia: is there any evidence? Crit Care 2014;18:210. [Crossref] [PubMed]
- Cai H, Gong H, Zhang L, et al. Effect of low tidal volume ventilation on atelectasis in patients during general anesthesia: a computed tomographic scan. J Clin Anesth 2007;19:125-9. [Crossref] [PubMed]
- Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care 2010;14:R1. [Crossref] [PubMed]
- Wrigge H, Uhlig U, Baumgarten G, et al. Mechanical ventilation strategies and inflammatory responses to cardiac surgery: a prospective randomized clinical trial. Intensive Care Med 2005;31:1379-87. [Crossref] [PubMed]
- Levin MA, Mccormick PJ, Lin HM, et al. Low intraoperative tidal volume ventilation with minimal PEEP is associated with increased mortality. Br J Anaesth 2014;113:97-108. [Crossref] [PubMed]
- Determann RM, Wolthuis EK, Choi G, et al. Lung epithelial injury markers are not influenced by use of lower tidal volumes during elective surgery in patients without preexisting lung injury. Am J Physiol Lung Cell Mol Physiol 2008;294:L344-50. [Crossref] [PubMed]
- Serpa Neto A, Hemmes SN, Barbas CS, et al. Protective versus conventional ventilation for surgery: a systematic review and individual patient data meta-analysis. Anesthesiology 2015;123:66-78. [Crossref] [PubMed]
- Manzano F, Fernández-Mondéjar E, Colmenero M, et al. Positive-end expiratory pressure reduces incidence of ventilator-associated pneumonia in nonhypoxemic patients. Crit Care Med 2008;36:2225-31. [Crossref] [PubMed]
- Serpa Neto A, Simonis FD, Schultz MJ. How to ventilate patients without acute respiratory distress syndrome? Curr Opin Crit Care 2015;21:65-73. [Crossref] [PubMed]
- Serpa Neto A, Hemmes SN, Barbas CS, et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med 2014;2:1007-15. [Crossref] [PubMed]
- Park SH. Perioperative lung-protective ventilation strategy reduces postoperative pulmonary complications in patients undergoing thoracic and major abdominal surgery. Korean J Anesthesiol 2016;69:3-7. [Crossref] [PubMed]


