Pathophysiological mechanism of post-lobectomy air leaks
Introduction
Postoperative air leaks are a common complication after lung resections. By conventional radiography, up to 40% of patients who undergo pulmonary resections will have a residual air space; three-quarters of these become spontaneously obliterated in the long term (1-3). The definition of prolonged air leak in the literature varies but is one that lasts more than either five or seven days (4,5).
Treatments available include the application of a blood patch or pneumoperitoneum, and intrabronchial and flutter valves. Possible surgical procedures include glue application, buttressing of staple lines using pericardium strips, pleural tenting, muscle flaps and cavernostomy (6,7). Pleural tenting reduces the chance of a postoperative air leak and shortens the duration of chest drain duration, decreases hospital stay, and prevents apical residual air spaces and their complications (8). Use of pericardial buttressing strips and collagen sponge coated with the human coagulation factors fibrinogen and thrombin is associated with a reduction in air leakage, earlier chest drain removal and decreased hospital stay (9). Improved surgical techniques and use of buttressing material have reduced but not abolished the risk of postoperative air leak (10). Postoperative air leaks are clinically important because they increase chest drain duration and hospital stay with an increased risk of space infection and higher hospital costs (11,12).
Upper lobectomy has been generally associated with a higher incidence of postoperative air leak (13,14), though not in all papers (15). Air leaks post-lobectomy have also been correlated with advanced age, low FEV1%, low BMI and the existence of pleural adhesions (15). Further, prolonged air leak may also be surgeon-dependent (3). Although it is understandable that poor quality lung as in emphysema is associated with an increased risk of postoperative air leak, it is not clear why different anatomical areas should suffer from the differing degrees of air leak when the lung parenchyma may appear macroscopically similar to a surgeon.
One answer to this question might be that different areas of the lung have differing levels of pleural stress, with pleural stress varying according to lung shape and location (16). As lung shape conforms closely to the form of the chest wall, differing chest wall shape may be associated with differing pleural stress extent. In a lung resection like a lobectomy, the remaining lung inflates into and conforms to the remaining space, taking up the shape of the resected lobe; this process occurs simultaneously with other physiological changes such as hyperinflation of the contralateral lung, mediastinal shifting, elevation of the ipsilateral diaphragm and ipsilateral crowding of the intercostal spaces (6).
Since it is impractical to directly measure pleural stress, an experimental finite element analysis (FEA) computer simulation was used. FEA modelling is an ideal method for calculating wall stress in pressure vessels with a complex shape. This method uses nodes in a mesh to model the original structure in three dimensions, reducing the complexity of the problem into a simpler solution with a finite number of mathematical calculations. Each node on the mesh has boundary conditions with the loads applied to it simulating the original loads.
Since the location of post-resection prolonged air leak remains contested (15), this paper aims to determine whether air leak following upper lobectomy was significantly higher than that following middle or lower lobectomy in a consecutive series of video assisted thoracic surgery (VATS) lobectomies from one centre. It also studies a model of pleural stress in the lung in order to correlate the location of postoperative air leak with pleural stress, since visceral pleural stress levels have already been implicated in the pathophysiology of primary spontaneous pneumothorax (PSP) as capable of generating parenchymal air leak on their own (16).
Method
Surgical patients
Between January 2014 and March 2017, data was prospectively collected from 367 consecutive VATS lobectomy resections using a fissureless technique without use of sealants, buttressed staple line or pleural tents, operated in a single institution in Ravenna, Italy. The chest tube management in this series included the use of one drain when appropriate, with suction not routinely used, following an enhanced recovery after surgery (ERAS) fast track program (17). A persistent air leak was defined as one lasting more than five days following recent publications rather than seven days as previously, although the number of patients at five days was identical to that at seven days in our case series (4,18,19).
Every variable was compared between upper versus middle/lower lobectomy. The data collected included age, gender and presence of pre-operative pathologies such as myocardial infarction (MI), congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease (COPD) defined following European Respiratory Society guidelines (20), connective tissue disease, peptic ulcer disease, diabetes mellitus, moderate to severe chronic kidney disease, hemiplegia, leukaemia, malignant lymphoma, solid tumour, liver disease, acquired immune deficiency syndrome (AIDS). Other data collected comprised the Charlson comorbidity index, the Eastern Cooperative Oncology Group (ECOG) score of performance status, forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEV1/FVC ratio or Tiffeneau-Pinelli index, diffusion capacity adjusted by the alveolar volume (DLCO/VA), preoperative blood gas analyser pO2, pCO2 and pH (partial pressure of oxygen, partial pressure of carbon dioxide, potential of hydrogen respectively), preoperative computerized tomography (CT) and positron emission tomography (PET) scan tumour sizing and staging and PET standardized uptake value (PET SUV). Further data collected included surgical technique (including operative time in minutes, lobe and side resected), pathological post-operative staging, length of postoperative hospital stay and duration of total hospital stay. Electronic meters to quantify air leak were not available.
FEA modelling
A finite element model of the lung was constructed with a first rib apical indentation. FEA is a method of solving complex structural analysis problems by simplifying the process through the use of a finite number of nodes that geometrically approximate the original shape.
The lung model created had height a of 24 cm, transverse radius b of 8 cm and anteroposterior radius c of 6 cm, see Figure 1, following methodology published by the authors (16,21). Meshing was performed using SOLID 187 element at a pressure of 40 kPa, with assumptions that the lung was elastic with a Poisson’s ratio of 0.3. FEA of the model was performed using Ansys v11 (ANSYS, Inc., Philadelphia, PA, USA) finite element simulation package using linear modelling. The base of the model was constrained superoinferiorly but allowed to inflate freely in the transverse and anteroposterior planes to mimic normal respiratory movements.

Statistics
The Chi-square test was used to examine the association between categorical variables and the Barnard’s test was explicitly used to examine 2×2 contingency tables using the facilities of R software (R Core team, Foundation for Statistical Computing, Vienna, Austria). Since some of the patients’ characteristics had a continuous metric scale and satisfied the normality assumption, the Independent samples t-test was used; where the Kolmogorov-Smirnov test indicated that the data was non-parametric, the Mann Whitney test was used instead. A binary Logistic regression model was used to relate the presence/absence of prolonged air leaks with baseline and postoperative patient characteristics (predictors) using a forward stepwise procedure to develop a parsimonious model using the facilities of SPSS software (IBM Corp, Armonk, NY, USA). A 0.05 level of significance was adopted for all statistical tests and models.
Results
Surgical patients
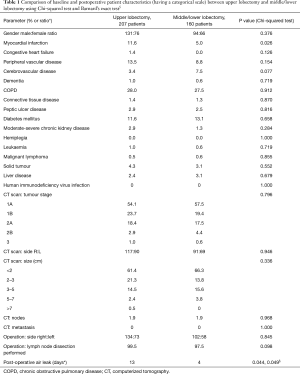
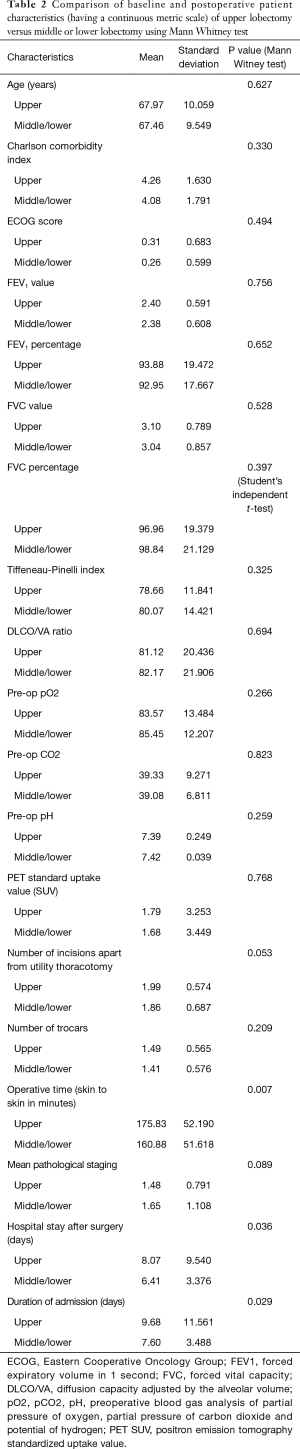
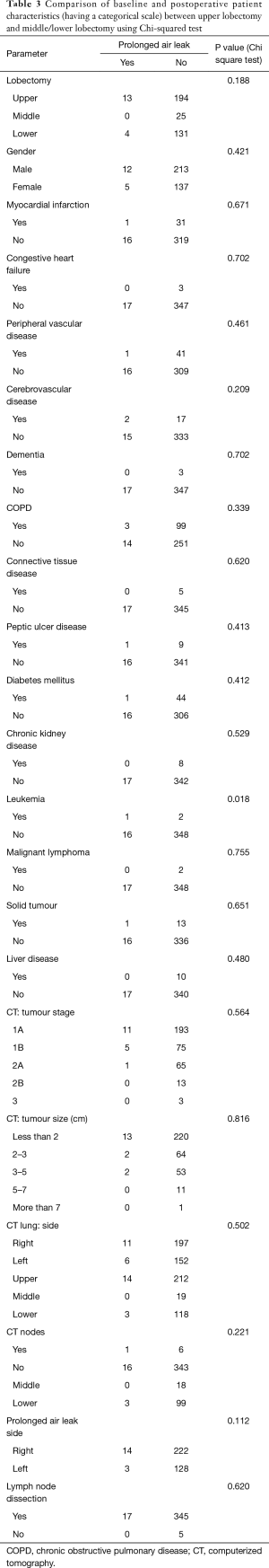
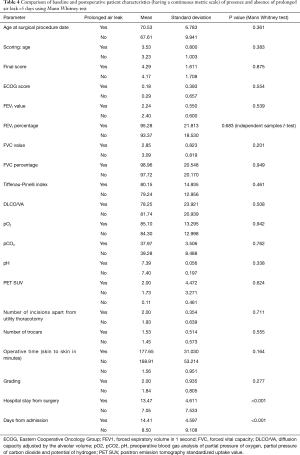
The percentage of cases with air leak following upper lobectomy (6.3%) was significantly higher than the percentage of cases with air leak following middle or lower lobectomy (2.5%), P=0.044 using Chi-square test and P=0.049 using Barnard’s test. Upper lobectomy was significantly more prevalent with a higher pre-operative MI rate (P=0.026), longer skin-to-skin operative time (P=0.007) and hospital stay (P=0.036). Other pre- and postoperative characteristics did not reach statistical significance, as displayed in Tables 1 and 2. Similarly all parameters were compared between presence and absence of prolonged air leak and the results are shown in Tables 3 and 4. The mean duration of hospital stay and mean days from admission were both approximately six days longer in cases of prolonged air leaks compared to cases without a prolonged air leak (P<0.001) with the mean duration of hospital stay and mean days from admission both approximately two days longer for upper lobectomy compared to middle or lower lobectomy (P=0.004). There was also an expected increase in hospital stay (P<0.001) and a medical history of leukemia (P=0.018, however numbers were very low). Interestingly there was a higher incidence of right-sided prolonged air leak (6.3%) versus the left-side (2.3%) but this did not reach statistical significance (P=0.112). The parsimonious logistic regression model showed no significant predictors apart from prolonged hospital stay, P=0.027. None of the patients with prolonged air leak patients required further interventional therapy.
Full table
Full table
Full table
Full table
Computational analysis FEA model
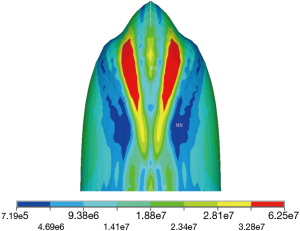
Stresses develop within the pleura on inflation, with the magnitude of the stress varying at differing levels of inflation e.g., breathing and coughing. There was an approximate eighty-fold difference in relative stress between the highest and lowest pleural stress levels in this model on 40 kPa maximal coughing, see Figure 2, with pleural stress highest near the apex and at the furrows for the first rib of the upper lobe and lowest at the base of the lower lobe, with air leak expected to be more likely to occur in the higher areas of pleural stress within the lung.
Discussion
The risk of a persistent air leak is associated with a low predicted postoperative forced expiratory volume in one second (FEV1), the presence of pleural adhesions, and an upper lobectomy or bilobectomy operation (22,23). Why is the presence of an air leak significant? It prolongs discharge from the hospital and increases the financial cost of the operation. Post resection residual pleural spaces also have a worse prognosis with older age, air leak or infection (14). This paper explores biomechanical stress levels in the pleura to explain why the rate of post-lobectomy air leaks differs in the different lobes.
Description of course of postoperative air space
A postoperative air space can be caused by the removal of a significant portion of the lung e.g. after bilobectomy resulting in reduced compliance and an inability to fill the residual space. It can also be due to leaks from the conducting zone e.g., broncho-pleural fistula, or the respiratory zone, like parenchymal leaks especially after extensive tissue dissection such as post-segmentectomy. Such spaces develop more commonly after upper lobectomy, at an apical location and on the right side especially in cases of lung cancer (14). A residual pleural space occurred in 40% after bilobectomy, 20% after lobectomy, and 5–10% after segmentectomy or wedge resection (2,13,23). Postoperative air spaces are in a dynamic state with approximately 20–40% of patient post-lobectomy having a residual air space; 75% of these become obliterated without intervention in the long term (8). Similarly, Solak reported an incidence at 40% on the first postoperative day decreasing to 10% at three months (4). The amount of air leak was related directly to the amount of suction applied and was least with a Heimlich valve (24).
Use of chest drains
Simple chest drains have been superseded by more costly electronic meters that digitally quantify the amount of air passing through chest tubes and can also measure pleural pressure; they were not available for the series of patients in this paper. An air leak meter incorporated in some triple compartment chest drain systems may indicate the approximate degree of air leak from the chest cavity; however, interpretation may vary between skilled personnel (25). Also, such systems did not change the duration of chest drainage or length of hospitalisation in a randomised trial (26) but diminished inert observer variability (27). However, it permitted distinguishing an actual air leak from a pleural space effect (28) and prediction of the duration of air leak (29).
Post-resection changes
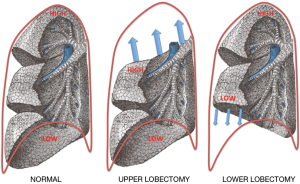
Removing an upper lobe leaves a space near the apex that takes time to decrease in size. The reason for this is that the remaining lung’s shape does not conform exactly to that of the rib cage and requires time before it can mould itself to match the chest wall. In an upper lobectomy, the exposed bullet shape of the apex of the lower lobe is expected to be subject to a much higher level of stress than the base of the lung extrapolating from our FEA model. In a lower/middle lobectomy, conformity between the remaining lung and chest wall is better, the apex remains in compliance with and supported by the chest wall, and this may decrease the chance of an upper air space, see Figure 3. After upper lobectomy, the bullet shape of the apex of the exposed lower lobe will be associated with high pleural stress, and a reduction in mechanical support by the chest wall to the visceral pleura due to initial post-op lack of chest wall confluence. It is suggested that the presence of higher stress levels in the lower lobe apex explains the higher parenchymal air leak post-upper lobectomy, in contrast with lower lobectomy where there is no remaining unsupported lung with high pleural stress levels.
Lung as pressure vessel
The lung when under internal pressure, for example during coughing or deep expiration will result in the lung acting as a pressure vessel, with Laplace’s Law describing how different areas of the visceral pleura are under different wall tension even though Pascal’s principle states that pressure is transmitted equally throughout the lung. Such differing wall tension or pleural stress has been used to explain the pathophysiology of spontaneous pneumothorax, with the apical area of the lung suffering from intense stress that induces tissues near the apex to tear apart and the pleura usually supported by the chest wall and by adhesions to the chest wall, thus relieving pleural stresses (16).
Once the lung is separated from the chest wall as occurs post-lobectomy, several factors are at play. One is that the lung expands in shape post-upper lobectomy to conform to the apical part of the chest wall, with expansion known to generate higher pleural stress. Another is that the remaining lung post-upper lobectomy has a shape where the apex of the upper lobe resembles a high pleural stress state. Another is that pleural pressures post-op tend to return to normal slowly and, since the pleural pressure is more negative at the apex than at the pulmonary base, lung parenchyma becomes exposed to a greater distending pressure differential than at the base of the lung. Further, the apical pleura in the presence of an apical air space lacks the structural support that would be provided by the chest wall should the lung be fully confluent. Thus after surgery, pleural forces do not remain unchanged but may increase in the postoperative period. The factors listed above may therefore explain why air leaks may develop or increase in the early days after an operation as the lung expands and why the presence of a pleural space would be expected to be associated with a greater risk of postoperative air leak.
Air space problems are commonest after upper lobectomies compared to other lobar resections (13), further confirmed in our series of 367 patients, P=0.044. The pressure vessel hypothesis, initially used to explain the pathogenesis of spontaneous pneumothorax (16) and later the reactivation of pulmonary tuberculosis (19), would suggest that the remaining lung post-upper lobectomy would be subjected to more stress because of the bullet shape of the exposed lower lobe and would therefore be expected to be at a higher risk of air leak. Pleural tenting, by covering the apex of the lung, would be expected to reduce pleural pressures by supporting the visceral pleura at the high-risk neo-apical area of the remaining lung, thereby reducing the rate of post-op air leak.
Right versus left lung air leaks
The value of predictions is that the scientific method is based on theoretical predictions that are logical consequences of scientific theories. The right lung being larger than the left would be expected to have higher stress on coughing or breathing as a pressure vessel. This would be predicted to result in a higher incidence of post-lobectomy prolonged air leak based on the hypothesis proposed here. Our data supports this prediction with a higher incidence of right-sided prolonged air leak (6.3%) versus the left-side (2.3%) but without reaching statistical significance (Chi-square P=0.112). Therefore the lowest air leak frequency would be predicted for left lower lobectomy (left lung being smaller and lower lobe location both leading to less pleural stress); this is supported by a report that air leak occurred less frequently after left lower lobectomy (P<0.001) (3).
Clinical correlates
This hypothesis explains why post-lobectomy air leaks are reduced by using methods that buttress the apical part of the remaining lung to reduce pleural stress or that change the shape of the pleural cavity by obliterating it, for example by using a pleural tent or even by using minimally invasive osteoplastic collapse thoracoplasty (31). Buttressing reduces pleural stress by supporting the pleura; in effect by increasing the thickness of the parenchymal pleura thus decreasing stress. Examples of buttressing include placing subcutaneous fat pads with fibrin glue in combination with a few mattress sutures (32) and glue or collagen sponge application. Theoretically, an apically located collagen sponge that changes the shape of the apex of the pleural cavity would be expected to reduce the incidence of air leaks by decreasing apical stress.
Other factors
Pleural stress has been associated with an anteroposterior flattened chest or low thoracic index (ratio of breadth to anteroposterior diameter of the chest) in both PSP and reactivation of tuberculosis (16,19). This pleural stress hypothesis would suggest that patients with a low thoracic index should be at a higher risk of postoperative air leak due to higher levels of pleural stress; this has yet to be confirmed or refuted. It may be argued that emphysema is known to affect the upper lobe more commonly than the other lobes (33), so stapling an emphysematous upper lobe may result in prolonged air leak; however, this was not a factor in our series P=0.912.
Conclusions
In this series of almost four hundred VATS lobectomies, air leak following upper lobectomy (6.3%) was significantly higher than that following middle or lower lobectomy (2.5%), resulting in a significant increase in a mean hospital stay of six days. A computer simulation FEA model of the lung indicates that the bullet-shaped apical region of the lung is prone to approximately eighty times higher stress than the lower part of the lung. This higher pleural stress manifests itself when there is a lack of lung-ribcage confluence and therefore support to the lung, as in the case of an apical air space as may occur after upper lobectomy when the highly stressed shape of the lower lobe apex is exposed in the initial post-op period. Such a mechanism may explain how why right-sided air leaks are commoner and why buttressing the apical portion of the remaining lung and changing the shape of the rib cage apex by use of a pleural tent decreases air leak. The pressure vessel hypothesis expounded here is consistent with known facts about post-lobectomy air leaks described above.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethics approval was obtained from the Institutional Review Board (No. 81/2014/O/Oss). The data was anonymously achieved according to the International Conference on Harmonization Guidelines for Good Clinical Practice. Informed consent for experimentation with human subjects was obtained from all individual participants included in the study. Privacy rights of the human subjects were always observed.
References
- Bell JW. Management of post-resection space in tuberculosis: II. Following lobectomy. J Thorac Surg 1956;31:442. [PubMed]
- Wareham EE, Barber H, McGoey JS, et al. The persistent pleural space following partial pulmonary resection. J Thorac Surg 1956;31:593-600. [PubMed]
- Okereke I, Murthy SC, Alster JM, et al. Characterization and importance of air leak after lobectomy. Ann Thorac Surg 2005;79:1167-73. [Crossref] [PubMed]
- Solak O, Sayar A, Metin M, et al. Definition of postresectional residual pleural space. Can J Surg 2007;50:39-42. [PubMed]
- Burt BM, Shrager JB. Prevention and management of postoperative air leaks. Ann Cardiothorac Surg 2014;3:216-8. [PubMed]
- Mueller MR, Marzluf BA. The anticipation and management of air leaks and residual spaces post lung resection. J Thorac Dis 2014;6:271-84. [PubMed]
- Nakada T, Inagaki T, Morikawa T, et al. Simplified Cavernostomy Using Wound Protector for Complex Pulmonary Aspergilloma. Ann Thorac Surg 2014;98:360-1. [Crossref] [PubMed]
- Okur E, Kir A, Halezeroglu S, et al. Pleural tenting following upper lobectomies or bilobectomies of the lung to prevent residual air space and prolonged air leak. Eur J Cardiothorac Surg 2001;20:1012-5. [Crossref] [PubMed]
- Anegg U, Lindenmann J, Matzi V, et al. Efficiency of fleece-bound sealing (TachoSil) of air leaks in lung surgery: a prospective randomised trial. Eur J Cardiothorac Surg 2007;31:198-202. [Crossref] [PubMed]
- Malapert G, Hanna HA, Pages PB, et al. Surgical sealant for the prevention of prolonged air leak after lung resection: meta-analysis. Ann Thorac Surg 2010;90:1779-85. [Crossref] [PubMed]
- Abolhoda A, Liu D, Brooks A, et al. Prolonged air leak following radical upper lobectomy: an analysis of incidence and possible risk factors. Chest 1998;113:1507-10. [Crossref] [PubMed]
- Varela G, Jiménez MF, Novoa N, et al. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:329-33. [Crossref] [PubMed]
- Barker WL. Natural history of residual air spaces after pulmonary resection. Chest Surg Clin N Am 1996;6:585-613. [PubMed]
- Misthos P, Kokotsakis J, Konstantinou M, et al. Postoperative residual pleural spaces: characteristics and natural history. Asian Cardiovasc Thorac Ann 2007;15:54-8. [Crossref] [PubMed]
- Brunelli A, Varela G, Refai M, et al. A scoring system to predict the risk of prolonged air leak after lobectomy. Ann Thorac Surg 2010;90:204-9. [Crossref] [PubMed]
- Casha AR, Manché A, Gauci M, et al. Is there a biomechanical cause for spontaneous pneumothorax? Eur J Cardiothorac Surg 2014;45:1011-6. [Crossref] [PubMed]
- Scarci M, Solli P, Bedetti B. Enhanced recovery pathway for thoracic surgery in the UK. J Thorac Dis 2016;8:S78-S83. [PubMed]
- Pompili C, Miserocchi G. Air leak after lung resection: pathophysiology and patients’ implications. J Thorac Dis 2016;8:S46-S54. [PubMed]
- Rivera C, Bernard A, Falcoz PE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg 2011;92:1062-8. [Crossref] [PubMed]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Series “ATS/ERS Task Force: Standardisation of lung function testing”. Eur Respir J 2005;26:948-68. [Crossref] [PubMed]
- Casha AR, Camilleri L, Manche A, et al. A hypothesis for reactivation of pulmonary tuberculosis: how thoracic wall shape affects the epidemiology of tuberculosis. Clin Anat 2015;28:614-20. [Crossref] [PubMed]
- Brunelli A, Monteverde M, Borri A, et al. Predictors of prolonged air leak after pulmonary lobectomy. Ann Thorac Surg 2004;77:1205-10; discussion 1210. [Crossref] [PubMed]
- Bell JW. Management of the postresection space in tuberculosis. III. Role of pre and postresection thoracoplasty. J Thorac Surg 1956;31:580-92. [PubMed]
- Anegg U, Lindenmann J, Matzi V, et al. AIRFIX®: the first digital postoperative chest tube airflowmetry—a novel method to quantify air leakage after lung resection. Eur J Cardiothorac Surg 2006;29:867-72. [Crossref] [PubMed]
- Zisis C, Tsirgogianni K, Lazaridis G, et al. Chest drainage systems in use. Ann Transl Med 2015;3:43. [PubMed]
- Lijkendijk M, Licht PB, Neckelmann K. Electronic versus traditional chest tube drainage following lobectomy: a randomized trial. Eur J Cardiothorac Surg 2015;48:893-8. [Crossref] [PubMed]
- McGuire AL, Petrcich W, Maziak DE, et al. Digital versus analogue pleural drainage phase 1: prospective evaluation of interobserver reliability in the assessment of pulmonary air leaks. Interact CardioVasc Thorac Surg 2015;21:403-7. [Crossref] [PubMed]
- Marasco RD, Giudice G, Lequaglie C. How to distinguish an active air leak from a pleural space effect. Asian Cardiovasc Thorac Ann 2012;20:682-8. [Crossref] [PubMed]
- Dernevik L, Belboul A, Rådberg G. Initial experience with the world’s first digital drainage system. The benefits of recording air leaks with graphic representation. Eur J Cardiothorac Surg 2007;31:209-13. [Crossref] [PubMed]
- Gray H. Gray's Anatomy. Philadelphia: Lea & Febiger, 1918.
- Krasnov D, Krasnov V, Skvortsov D, et al. Thoracoplasty for Tuberculosis in the Twenty-first Century. Thorac Surg Clin 2017;27:99-111. [Crossref] [PubMed]
- Shintani Y, Inoue M, Nakagiri T, et al. Use of free subcutaneous fat pad for reduction of intraoperative air leak in thoracoscopic pulmonary resection cases with lung cancer. Eur J Cardiothorac Surg 2014;46:324-6. [Crossref] [PubMed]
- Takahashi M, Fukuoka J, Nitta N, et al. Imaging of pulmonary emphysema: A pictorial review. Int J Chron Obstruct Pulmon Dis 2008;3:193-204. [Crossref] [PubMed]


