A case of bronchial artery aneurysm with an esophageal fistula as an extremely rare complication after bronchial arterial embolization
Introduction
A bronchial artery aneurysm with an esophageal fistula (BAAEF) is an extremely rare and potentially fatal condition. Only three cases of a bronchial artery aneurysm (BAA) with hematemesis have been reported previously. Two cases of the pinhole-type were successfully treated with only coil embolization, while one case was lost due to massive bleeding (1-3). Here, we report a case of a BAAEF that developed 3 months after bronchial arterial embolization (BAE) for hemoptysis.
Case presentation
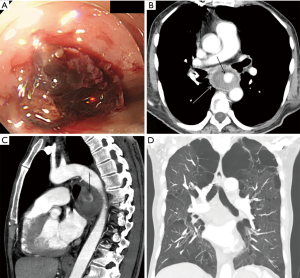
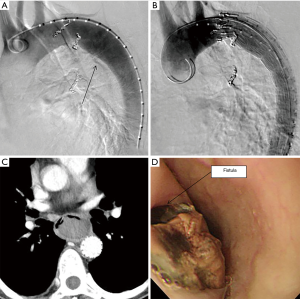
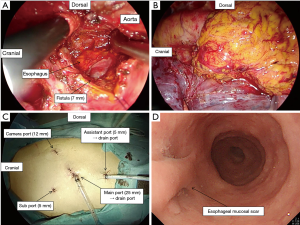
A 67-year-old man was referred to our institution with hematemesis. He had undergone emergent BAE for massive hemoptysis under artificial ventilation 3 months previously at another hospital. He had severe asthma–chronic obstructive pulmonary disease overlap syndrome and uncontrolled diabetes mellitus. His vital signs were stable. His white blood cell count and serum C-reactive protein level were within the normal range, but his hemoglobin level (8.7 mg/dL) was low. Emergent upper gastrointestinal endoscopy revealed an extrinsic mass in the esophagus with oozing blood without an obvious esophageal fistula at 33 cm from the incisors (Figure 1A). We needed a further examination of the extrinsic mass in the esophagus as the source of bleeding. Enhanced chest computed tomography (CT) showed a 5-cm saccular aneurysm with enhancement adjacent to the descending thoracic aorta and severe emphysema (Figure 1B,C,D). We identified the esophageal extrinsic mass as the saccular aneurysm because of the similar position. The previous chest CT and angiography at the BAE revealed no aortic or bronchial arterial aneurysm. Because the descending aorta was normal on CT, we made a diagnosis of iatrogenic BAA caused by BAE. Because the neck of the aneurysm was too short to treat with transcatheter coil embolization (Figure 2A), we performed emergent thoracic endovascular aortic repair (TEVAR) using the Zenith® TX2 stent graft (ZTEG-2PT-32-160-PF, Cook Medical Inc, Bloomington, IN, USA) under general anesthesia to prevent critical bleeding (Figure 2B). The aneurysm was successfully excluded and hemostasis was achieved. After this emergent surgery, we had planned elective surgery for the esophagus. But, he had not enough condition to tolerate general anesthesia because of the left acute pneumonia. On day 13 after the operation, despite ingestion limitation, enhanced chest CT revealed reduction of the size of the aneurysm, however, an air density appeared inside the excluded aneurysm. Furthermore, esophageal endoscopy showed round mucosal degeneration of 2 cm in diameter with a fistula leading towards the aneurysm (Figure 2C,D). He recovered the left acute pneumonia by antibiotics, and then, his cardiac ejection fraction was 46.8% and forced expiratory volume in 1 second as a percentage of forced vital capacity was 44%. We decided to perform minimally invasive surgery because of his poor condition. We performed aneurysmotomy, debridement, and pedicled omental flap installation between the fistula and the descending thoracic aorta using a left thoracoscopic approach (Figure 3A,B,C). An enterostomy tube was inserted for nutritional support, and he had been controlled adequate glycemic control by a diabetes specialist. Cultivation test of the removed hematoma was positive for Pseudomonas aeruginosa. The patient developed left pyothorax and on day 14 after the second operation, we performed a left single portal thoracoscopic debridement and drainage via a 2.5 cm skin incision under local anesthesia. Follow-up esophagography and esophageal endoscopy showed healed fistula without stenosis (Figure 3D). He started oral intake on day 15 after the third operation, and the enterostomy tube was removed. He was discharged home on day 71 after the first operation. He was able to resume a normal life and was able to be reinstated 2 weeks following hospital discharge. He had been treated with oral levofloxacin for preventing the infection of stent graft for 1 year. Four months after the medication, he continues to do well.
Comments
A BAA is a rare and potentially fatal condition (4). Moreover, BAAEF is an extremely rare condition. We could clinically diagnose the aneurysm as iatrogenic BAA to consider the clinical and imaging course, and supposed a pseudoaneurysm as an iatrogenic aneurysm caused by BAE. However, we could not pathologically distinguish the aneurysm between pseudoaneurysm and primitive degenerative aneurysm because of no evidence for pathological finding. We reviewed the literature for “bronchial arterial aneurysm”, “bronchial arterial pseudoaneurysm”, “bronchial arterial embolization”, “esophageal fistula”, “iatrogenic”, “complication”, “gastrointestinal bleeding”, “esophageal perforation”, and “hematemesis” using PubMed and found only three cases of a BAA with hematemesis. Only our case was caused by iatrogenic complication. Some authors had reported critical complications caused by BAE, including bronchial ischemia/necrosis, bronchoesophageal fistula, spinal cord injuries, and embolization of other vessels (5-9). As same iatrogenic condition like BAAEF, Wiegand reported an iatrogenic aorto-esophageal fistula caused by vascular plug for an abnormal artery originated from the descending aorta (10). Shaer reported the first case of a BAAEF. The patient had a 6-mm perforation and died of massive hematemesis within 12 hours of the first symptom (1). Two other BAAs of a pinhole-type have been reported, and these cases were successfully treated with only coil embolization without aneurysmal infection (2,3). In our case, we could diagnose a delayed-onset BAPEF on follow-up CT and esophageal endoscopy. Hematemesis due to pinhole-type BAA without infected aneurysm or blood circulation disorder of the esophagus may be cured without an esophageal fistula. The patient should be followed up with periodic CT or endoscopy, and if BAAEF develops, definitive procedure such as shown in this article should be considered. BAAEF is also rare condition, but should be recognized as a potential complication following BAE.
The etiology of a BAA has not been established. Open surgical repair may have high morbidity and mortality rates if the patient has a poor condition, and treatment may be difficult if the aneurysm is located close to the thoracic aorta (11). Kasashima reported a successful treatment of a BAA with TEVAR without embolization of the BAA or the distal bronchial artery (4,12). TEVAR is indicated for BAA with tortuous, large, and short neck, which is difficult to treat with transcatheter coil embolization. In our case, emergent TEVAR successfully prevented critical bleeding.
Esophagectomy is may be too invasive for high-risk patients. Omura reported successful treatment of an aorto-esophageal fistula using pedicled omental flap repair without esophagectomy; however, the patient died from aspiration pneumonia 5 months after thoracotomy (13). In our case, the size of the fistula was 7 mm, and we performed pedicled omental flap repair without closing the hole. We consider that pedicled omental flap repair is an effective and less invasive treatment for esophageal perforation. Invasive open thoracic surgery not only has a high risk of early and late fatal pulmonary complications, but may also impair quality of life (QOL). Thoracoscopic surgery can overcome these problems (14). We believe that our thoracoscopic approach prevented short and long-term respiratory complication and enabled the patient to maintain QOL and resume a normal life despite his poor cardiopulmonary function.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Witten informed consent was obtained from the patient for the writing of this manuscript.
References
- Shaer AH, Bashist B. Computed tomography of bronchial artery aneurysm with erosion into the esophagus. J Comput Assist Tomogr 1989;13:1069-71. [Crossref] [PubMed]
- Kim JS, Lee SY, Son KH, et al. Bronchial Artery Aneurysm Presenting as Hematemesis and Mediastinal Hemorrhage. Korean J Thorac Cardiovasc Surg 2015;48:298-301. [Crossref] [PubMed]
- Fukunaga A, Okushiba S, Ohno K, et al. Mediastinal bronchial artery aneurysm with hematemesis. Dis Esophagus 2003;16:328-31. [Crossref] [PubMed]
- Takahashi Y, Tsutsumi Y, Monta O, et al. Stent grafting for giant bronchial artery aneurysm disguised as an aneurysm with multiple feeding arteries. Ann Thorac Surg 2010;89:1652-4. [Crossref] [PubMed]
- Pestana Knight EM, Novelli PM, Joshi SM. Cerebral and systemic infarcts after bronchial artery embolization. Pediatr Neurol 2011;45:324-7. [Crossref] [PubMed]
- Peynircioglu B, Ergun O, Hazirolan T, et al. Bronchial to coronary artery fistulas: an important sign of silent coronary artery disease and potential complication during bronchial artery embolization. Acta Radiol 2007;48:171-2. [Crossref] [PubMed]
- Hsu HK, Su JM. Giant bronchoesophageal fistula: a rare complication of bronchial artery embolization. Ann Thorac Surg 1995;60:1797-8. [Crossref] [PubMed]
- Girard P, Baldeyrou P, Lemoine G, et al. Left main-stem bronchial stenosis complicating bronchial artery embolization. Chest 1990;97:1246-8. [Crossref] [PubMed]
- Munk PL, Morris DC, Nelems B. Left main bronchial-esophageal fistula: a complication of bronchial artery embolization. Cardiovasc Intervent Radiol 1990;13:95-7. [Crossref] [PubMed]
- Wiegand G, Schlensak C, Hofbeck M. Esophageal perforation caused by an AMPLATZER(TM) vascular plug 4 occlusion device. Catheter Cardiovasc Interv 2016;88:E113-6. [Crossref] [PubMed]
- Kim YK, Sung YM, Kim JH, et al. Aortic stent-graft for a giant bronchial artery aneurysm with ultrashort neck. Ann Thorac Cardiovasc Surg 2014;20 Suppl:781-5. [Crossref] [PubMed]
- Kasashima F, Endo M, Kosugi I, et al. Mediastinal bronchial artery aneurysm treated with a stent-graft. J Endovasc Ther 2003;10:381-5. [Crossref] [PubMed]
- Omura A, Yoshida M, Koda Y, et al. Surgical management without resection of the oesophagus for aorto-oesophageal fistula secondary to aortic arch aneurysm rupture. Interact Cardiovasc Thorac Surg 2016;23:985-7. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]


