Pneumothorax and asthma
Asthma
Bronchial asthma is a chronic inflammatory disease of the airways (1) caused by a combination of genetic and environmental factors (2) producing a range of pathogenesis and severity. There is a continuous increase in the prevalence of asthma worldwide causing a major public health concern. Bronchial asthma is still the most common chronic disease of childhood (3) and one of the leading causes of morbidity in children worldwide. In addition, children’s inability to express warning signs and seek medical attention in a timely manner makes the management of asthma in children a challenge.
Airway inflammation is one of the pathophysiological characteristics of asthma whose role is currently well known. Airway inflammation is mediated through infiltration of inflammatory cells, including mast cells, eosinophilic and neutrophilic granulocytes in the airway wall (4). This cell infiltration subsequently leads to bronchial hyperresponsiveness (BHR) and persistent changes of the airways in case of chronic inflammation i.e., airway remodeling (4,5).
Symptom evaluation is the key to diagnosis and outcome measures in clinical studies (6). Repeated episodes of wheeze, dyspnea, chest tightness and cough particularly at night or early in the morning include the main symptoms of asthma (7). These episodes are usually associated with extensive but variable obstruction of air flow inside the lungs, which is often reversible, either spontaneously or after treatment. Wheezing seems to be the most important symptom in identifying asthma (8).
Diagnosis of asthma is based on the measurement of lung function using spirometry (7). After the diagnosis of asthma is established, the next step is the management of the disease. Medical therapy involves two different classes of medication: inhaled corticosteroids used as daily controller and β andrenergic agonists used for bronchodialation (1). Although the last two decades asthma treatment is based on the systematic use of anti-inflammatory therapy, recently, important issues regarding the management of asthma include early diagnosis and implementation of the appropriate therapy along with the development and approval of novel medications. In addition, recent studies have focused their research on the identification of asthma phenotypes/endotypes, suggesting phenotype-specific treatment (9,10).
Except from the development of the airway inflammation during an asthma attack, the simultaneous bilateral spontaneous pneumothorax (SBSP), the subcutaneous emphysema and the pneumomediastinum are life threatening complications which may appear separately or rarely simultaneously.
Pneumothorax
Pneumothorax represents a common clinical problem and is categorized either to spontaneous pneumothorax (SP) (primary and secondary or catamenial) or nonspontaneous such as traumatic with iatrogenic or no iatrogenic etiology (11,12). It was first recognized in 1819 (13) and in 1850 was established as a complication of childhood asthma (14).
More specifically, primary spontaneous pneumothorax (PSP) is defined as the spontaneously occurring presence of air in the pleural space in patients without clinically apparent underlying lung disease, whereas a secondary spontaneous pneumothorax (SSP) is defined as a SP with underlying lung disease (12) including cystic fibrosis, chronic obstructive pulmonary disease (COPD), AIDS, etc. In patients with SBSP, the incidence of an underlying lung disease is greater than in the patients with unilateral SP (15). Τhe ratio of SBSP is approximately 1.3% among all the cases of pneumothorax (16). Another common term, often used in literature by physicians, includes the term of tension pneumothorax. According to Leigh-Smith et al., tension pneumothorax is defined as “pneumothorax with significant respiratory or hemodynamic compromise that reverses on decompression (needle or finger) alone” (17) i.e., the improvement occurs before one way valve drainage is connected. Thus, there are two distinct epidemiological types of SP: PSP and SSP. PSP has a peak incidence in young people while SSP has a peak incidence at ages of 55 years (18). PSP remains a significant health problem with an annual incidence of 18-28 per 100,000 population in males (19) and 1.2-6.0 per 100,000 population in females (15). Another well-known risk factor for PSP incidence regarding both genders is smoking (20).
It is a fact that the pathophysiology of pneumothorax remains poorly understood. Currently, a combination of emphysema-like changes, which are blebs and bullae, and abnormalities in the structure of the lung called pleural porosity are believed to cause pneumothoraces (21). The issue is the spontaneous occurrence of a communication between alveolar spaces and the pleura. In humans, the right and left pleural spaces are completely separated without evidence of preformed anatomic communications (22). Although intrapleural pressures are negative throughout most of the respiratory cycle (23), air does not enter into the pleural space because the sum of all the partial pressures of gases in the capillary blood averages only 93.9 kPa (706 mmHg). As a result, net movement of gases from the capillary blood into the pleural space would require pleural pressures lower than –54 mmHg (i.e., lower than –36 cm H2O), which hardly ever occur in normal circumstances (24). Hence, if air is present in the pleural space, any of the following has occurred: (I) communication between alveolar spaces and pleura; (II) direct or indirect communication between the atmosphere and the pleural space or (III) presence of gas-producing organisms in the pleural space.
There is low morbidity and no mortality when PSP is properly treated as the clinical manifestations and treatments of PSP are well-documented and established.
As the course of SP remains unpredictable with a recurrence rate ranging 25-54% (25), there are many options in the management of SP. The treatment of pneumothorax focuses on evacuating air and on preventing recurrence. Several therapeutic approaches have been developed for the treatment of PSP including conservative (observation or aspiration) or more invasive therapeutic options such as thoracotomy and VATS (20). In the first episodes of PSP, there is no doubt that observation and simple aspiration were established first-line therapies, as reported in randomized controlled trials (15,24,26). Observation therapy can be appropriate only for the patients who had mild (less than 20%) unilateral pneumothorax but mortality rate can reach 5% for such cases due to tension pneumothorax during observation therapy (27). The treatment to prevent recurrence of pneumothorax is mandatory in clinical situations such as SSP with potential respiratory insufficiency, recurrent PSP or persistent pneumothorax. There are two main options for SSP treatment, either medical talc pleurodesis by simple thoracoscopy or surgical approach (28). Although chemical pleurodesis can treat complicated or recurrent pneumothorax, surgical treatment modalities are more efficient. Chemical pleurodesis is only applied when surgical procedure cannot be performed on the patient.
SP may be the initial presentation in a wide variety of disorders (29-31), thus, it is most likely that several different mechanisms are responsible for a common result. Recently, in the effort to identify pathologic features which may correlate to specific clinical syndromes, the researchers reviewed 92 cases of SP (primary and secondary) regardless the etiology during a 11-year period (32). Their results identified a distinct pattern of pneumothorax-associated fibroblastic lesions in a subset of cases of SP while further studies need to shed light whether this is related to the pathogenesis of the pneumothorax.
Pneumomediastinum
Pneumomediastinum is defined as the presence of free air in the mediastinum. Pneumomediastinum can be divided into spontaneous pneumomediastinum (SPM) without any obvious primary source and into secondary or traumatic pneumomediastinum with mediastinal organ injury or other known precipitating events such as trauma, surgery or medical procedures. According to Macklin’s experimental animal model, alveolar rupture leads to air infiltration along the bronchovascular sheath with free air finally reaching the mediastinum. Furthermore, if the air travels along tissue planes and reaches the neck, face, abdomen or even the limbs, this can lead to subcutaneous emphysema (33). SPM was originally described by Louis Hamman in 1939 (34), while later it was identified as Hamman’s syndrome. Moreover, the crepitus heard-a peculiar and distinctive crunching sound with the heartbeat on chest auscultation mainly during systole but also in diastole is known as the Hamman sign. On the other hand, traumatic pneumomediastinum was first described by Laennec in 1819 in a 4-year-old boy who suffered a car accident (35).
Some studies demonstrate that SPM is presented equally in men and women (36-38), while others suggest that occurs mostly in men (39-41). In general, studies support that this condition is presented mostly in healthy young adults (36,37,39,40), especially in the presence of asthma (22%). According to Abolnik et al. the incidence of SPM is approximately one in 33,000 in the general population and one in 25,000 in the age group of five to 34 years (42).
Sudden onset of symptoms of SPM include retrosternal pain (80%), rhinophonia and/or hoarseness (65%), dyspnea (46%), cough (26-45%), subcutaneous emphysema (32%), sore throat (18%), and neck pain (4-38%) (43). According to several reports, the most common presenting complaints are chest pain, shortness of breath and subcutaneous emphysema (39,40,44). Emphysema, asthma, interstitial lung diseases, and bronchiectasis are some structural lung diseases that may predispose patients to the development of SPM (45-47). Although pneumomediastinum is a rare condition, it may occur in up to 10% of patients with cercical trauma and severe blunt thoracic trauma which includes the most common injury causing secondary pneumomediastinum (48). Moreover, forceful staining during exercise, diabetic ketoacidosis, childbirth, severe cough and vomiting, inhalation of drugs such as marijuana and cocaine (48), emesis (39), intense screaming (38) include known conditions that have been associated with increased pressure in pneumomediastinum. However, several reports agree upon the absence of a triggering event responsible for SPM in a significant number of patients (36). As most of the chronic bronchitis patients are young with non-specific symptomatology, they usually seek medical attention 24-48 hours after the appearance of symptoms.
Overall, pneumomediastinum is a clinical condition that is treated according to the age, the clinical status and the underlying aetiology. Although it is usually benign in young patients, it may become life-threatening in advanced ages and in patients with limited pulmonary reserve, unless it is urgently managed.
Banki et al. in order to assess the etiology of pneumomediastinum and identify predictive factors of mediastinal organ injury such as the esophagus or trachea injuries, included 449 patients with trauma and nontrauma pneumomediastinum (48). According to previous studies, mediastinal organ injury is uncommon in patients with pneumomediastinum (49-51). Similarly, Banki et al. concluded that patients presented with pneumomediastinum without a history of instrumentation, pleural effusion or vomiting, usually, do not have mediastinal organ injuries.
Early suspicion by the clinician is the answer key to the diagnosis preventing devastating changes in the cardiorespiratory dynamics supported by mechanical ventilation (52). Diagnosis can be established clinically and radiologically while Hamman’s sign is occurring (44). It is not clear yet the usefulness of diagnostic testing in determining a cause for SPM.
The diagnosis of pneumomediastinum is based upon radiographic examination, favoring CT chest scan to plain chest X-ray film (41). Caceres et al. reported that plain chest X-ray film was shown to be more sensitive for the diagnosis of pneumothorax and pleural effusion than for pneumomediastinum. Additionally, subcutaneous air was more frequently seen by CT scan in secondary pneumomediastinum as compared with SPM.
According to some cases (43) conventional X-rays in two projections might be negative in up to 30% of patients. Furthermore, inexperienced primary care physicians may easily overlook radiographic findings of SPM; however, computed tomography is now the golden standard procedure for the diagnosis of pneumomediastinum. According to several cases (53), esophagography, bronchoscopy, and esophagogastroduodenoscopy which include additional diagnostic testing, have shown limited results in the evaluation of SPM patients and are unnecessary in most cases.
In the early stages, when pneumomediastinum appears in the so-called occult form may not be detected with conventional radiography, particularly in cases of underlying chronic condition such as allergic asthma especially if it is masked by fever. According to Russo et al., the use of ultrasound in Emergency Department enabled the rapid diagnosis of the two pneumomediastinum cases (43), suggesting that the integration of conventional radiography with thoracic ultrasound might reduce radiobiological risk.
The challenge concerning the treatment of pneumomediastinum includes the distinction of the patients with mediastinal organ injuries who probably require surgical treatment from the patients without organ injuries who can simply be monitored. Rest, analgesics, antibiotics (for bacterial infections only) and clinical monitoring consist the conservative symptomatic treatment (54,55) for SPM which is applied very quickly (24-48 hours). In case of concomitant pleuroparenchymal conditions, the clinical course may be longer with obvious repercussions for recovery as seen in a 26-year-old chronic asthma patient (43). In contrast, secondary pneumomediastinum requires surgical repair of the post-traumatic or iatrogenic bronchial or esophageal defect causing air infiltration, and has a poor prognosis in the case of occult lesions. Additionally, persistent entry of air into the mediastinal leads to tension pneumomediastinum which can impair venous return, thus air should be promptly evacuated via either percutaneous methods or a surgical approach (56).
Overall, the mainstay of therapy is based on the treatment of the underlying cause; however, precipitating factors should be identified and controlled to prevent recurrence. The first case of recurrence SPM was reported by Yellin and colleagues (57) in 1983. Since then, recurrence of SPM has been rarely reported. Recently, the recurrence of SPM after a free-interval of 12 months was presented in a 21-year-old male with a mental deficiency (58).
Asthma and pneumothorax
The relationship between asthma and pneumothorax needs to be established. A report more than 30 years ago in asthmatic patients admitted to hospitals in central England concluded that a SP was uncommon (14). More recently, Tanaka et al. reported that among 67 patients with SSP in Japan, asthma was confirmed in only two patients (59). Similarly, among SP patients in USA, it was found that only four out of 45 patients with SSP had asthma (27). All these previous reports emphasize that SSP is an uncommon complication of asthma; however an equivalent percentage of asthma development corresponds to the general population.
Although the association between SSP and COPD is well-known, the association between SSP and asthma is less known (60). The clinical presentation of SSP may be similar to that of asthma deterioration, therefore, the diagnosis of SSP may be often delayed or missed (61). Both tension pneumothorax and asthma are manifested by respiratory distress, wheeze, tachycardia, tachypnea, desaturation, hyper-expansion, agitation and decreased air entry, causing difficulty in achieving an accurate differential diagnosis. As tracheal deviation and venous distension are unreliable signs and hypotension is extremely rare in the unventilated patient (17), chest pain and ipsilateral hyper-resonance can only point towards a diagnosis of tension pneumothorax which may also be difficult to differentiate in an anxious hypoxic patient. Furthermore, hyper-expansion would be expected to be bilateral in asthma whereas in tension pneumothorax would be ipsilateral hyper-expansion with hypo-mobility and contralateral hyper-mobility; however, this distinction requires a detailed identification.
Overall, in asthmatic patients, a finding of decreased air entry on chest auscultation and symmetrical or focal findings may suggest pneumothorax. In patients with increased shortness of breath and chest pain, a chest radiograph should be performed. Although the chest pain is present in most PSP cases, it is less common in patients with SSP (62). Therefore, it is essential that all patients admitted to hospital with acute asthma, after the assessment of severity and appropriate treatment, should have a chest radiograph to exclude a clinically unsuspected pneumothorax as the cause of deteriorating asthma. Although the chest X-ray is a standard procedure, thoracic tomography gives superior results in terms of small pneumothorax. The radiological signs of partial pneumothorax depend upon its volume, its topography, the patient’s position, and the previous existence of pleural or pulmonary disease. It is a fact that asthma predisposes susceptible individuals to pleural bleb rupture and pneumothorax when bronchospasm and hyperinflation increase the pleural pressures needed to maintain ventilation.
There are a few cases in literature which present asthmatic patients with pneumothorax. One case in England included a 19-year-old male with 12-year history of asthma, who required several hospital admissions in order to treat his acute asthmatic attacks, none of which was complicated by a pneumothorax (63). However, in a later admission for acute dyspnea, his chest radiograph revealed a large right pneumothorax with collapse of the right lung. He was treated with intercostal tube drainage several times, but his X-ray revealed a large air cyst in the right lower zone. The patient underwent a right thoracotomy to remove the cyst and a pleurodesis to control the pneumothorax. Although the cause of this encysted pneumothorax was unclear, the physicians assumed that pleural adhesions were a possible explanation.
Another asthmatic 61-year-old man was admitted to a clinic with haemoptysis describing a left chest pain during inspiration (64). The CT scan showed an emphysema and a pneumothorax which was confined only interlobar and disappeared after three weeks. Spontaneous interlobar pneumothorax includes an unusual topographical feature of pneumothoraxes (65-67) and was originally described by physiologists (4) as a complication of tuberculosis. A few cases of interlobar pneumothorax have been observed by thoracic surgeons following pleurodesis (67) but it does not occur without pleural adhesion. More recently, another case reported a 29-year-old asthmatic male with shortness of breath which was continually deteriorated for three days despite his medication with inhaled short-acting B2-agonist and inhaled corticosteroid (61). Chest X-ray examination confirmed the presence of pneumothorax on the left side, which occupied 60% of the space within the pleura on expiration. The treatment included a left tube thoracostomy.
The first case of bilateral localized tension pneumothorax was reported in the pediatric age group. This case of a 12-year-old asthmatic child with bilateral localized tension pneumothorax was not only difficult to diagnose but also refractory to needle decompression (68). Similarly, a rare case of a simultaneous spontaneous bilateral pneumothorax (SSBP) in a 14-year-old asthmatic female was demonstrated by Williams-Johnson et al. (69). While the patient received nebulizations, developed suddenly supraclavicular fullness with crepitus and bilateral needle thoracocentesis in the second intercostal spaces were performed. This case illustrated an unusual sudden complication of an asthmatic attack showing that even though SSBP is associated more with patients on mechanical ventilation, physicians must be aware of the disease’s progression in order to initiate early and aggressive lifesaving therapy. Possible explanations of this condition include the severe expiratory obstruction with air trapping, the rupture of a large mediastinal subpleural bulla on one side resulting in so called “buffalo chest” and a pre-existing anatomical single pleural cavity (70). It was Schorlemmer et al. who referred the absence of anatomical separation of the two hemithoraxes, the “buffalo chest” condition (71), as the North American buffalo or bison is one of the few mammals to have a single pleural space. Bilateral SP is a rare condition and may be secondary to chronic diseases such as asthma or COPD, as it might be for this case. Given that SP is often related to cystic fibrosis, Flume et al. reported that SSBP is also often associated with cystic fibrosis (72). However, in this case there was no family history or any systemic symptoms and signs to suggest cystic fibrosis.
Although SSBP is an uncommon situation among all the cases of pneumothorax, a 19-year-old man was diagnosed with SSBP and symptoms suggestive of occupational asthma (73). Video-assisted thoracoscopic surgical technique, bilateral bulbectomy and pleurodesis were performed, however, the pneumothorax reoccurred unilaterally and open surgery was also performed.
Recently, a case of tension pneumothorax in an 86-year-old woman who underwent cataract surgery was associated with asthma attack during general anesthesia suggesting that the cause of the tension was possibly due to the hyperinflation of fragile alveoli by the mechanical ventilation (74). Although the exact etiology of the bilaterality of this SP was not identified, the case demonstrated an unusual sudden condition which required early identification and appropriate treatment. Possible explanations of this condition include the severe expiratory obstruction with air trapping, a pre-existing anatomical single pleural cavity (buffalo chest) and the possibility of bilateral localized tension pneumothorax, however, this would be difficult to diagnose due to the lack of asymmetry in the physical findings (69).
Lung volume recruitment (LVR) is a dynamic physiologic process which is used to reopen collapsed aveoli and increase peak cough flow in order to maintain airway patency and improve ventilation (75). Air stacking uses a manual resuscitator to achieve maximum insufflation capacity in patients with respiratory muscle weakness. It is a technique considered to be safe with rare reported complications (76,77). Air stacking was performed for three years in a 72-year-old woman who had periodic but infrequent asthma attacks. The patient suddenly developed a pneumothorax indicating that the technique should not be used or used with caution in patients with a known pulmonary pathology (78). Chest tube drainage was immediately occurred resulting in successful extubation and four days later as the lung expanded, talcum pleurodesis was performed. Therefore, especially elderly patients who may have an undiagnosed pulmonary pathology and those with medical history of pulmonary diseases should be warned about the possible complications of LVR, while diagnostic imaging of the chest should be considered prior to LVR.
In another report, a middle-aged female patient with tuberous sclerosis presenting with SP secondary to lymphangioleiomyomatosis, was previously mistaken for asthma (79). Her acute management included lung re-expansion via chest tube insertion, antibiotics for concurrent chest infection, nebulisation and chest physiotherapy. Since hospital discharge, the patient had only occasional shortness of breath relieved by bronchodilators.
A 21-year-old man with a history of bronchial asthma during childhood presented with left recurrent pneumothorax (80). Thoracoscopic bullectomy and pleurodesis were performed. Lung ventilation scintigram which was performed during the absence of pneumothorax showed low accumulation and delay of washout in the left upper lobe. These findings are compatible with Swyer-James syndrome. According to the literature, several cases of SP have been reported in patients with Swyer-James syndrome (81,82), in which air leakage was found from the bullae in the diseased lung. Researchers suggested that in order to prevent the recurrence of pneumothorax related to Swyer-James syndrome due to emphysematous changes of the diseased lung, a procedure such as extensive pleurodesis is necessary (80).
Furthermore, even though the majority of deaths from asthma occur in chronic and severe asthmatic patients, some deaths occur suddenly in patients with moderate or mild asthma (83). Tension pneumothorax as the cause of death may be missed, unless post mortem examination includes chest radiology or monitors pleural pressure before opening the thoracic cavity (84,85). This raises the question as to whether any asthma deaths may be due to tension pneumothorax and if they could have been prevented. However, as asthma is a common disorder, pneumothorax and asthma can be coincidentally related rather than causally.
Overall, asthma is one of the most common chronic diseases and the morbidity and mortality is high when is associated with SSP. Physicians must always have a high index of suspicion in diagnosis of asthmatic patients who appear with suggestive symptoms or focal chest findings. The higher risk of coincidental pneumothorax in asthmatic patients is the unilateral focus on asthma and consequently the delayed diagnosis.
Pneumomediastinum, pneumothorax and asthma
Although pneumomediastinum is generally a benign and self-limiting condition that responds to conservative therapy, its concurrence with pneumothorax may prove fatal during a serious asthma attack. Some serious complications of pneumomediastinum include high blood pressure and/or bilateral pneumothorax, as well as cardiac compression and high pressure causing a reduction in the cardiac output. In severe acute asthma, the overexpansion of the distal air ways due to the obstruction in the minor air ways and the subsequent alveolar rupture, are responsible for the development of the pneumomediastinum. Due to the pressure difference, the air in the pulmonary interstitium moves in the centripetal direction from the pulmonary parenchyma towards the mediastinum which explains the occurrence of pneumomediastinum (86).
There is a 10% probability of pneumomediastinum to evolve to pneumothorax while the converse never happens. As misdiagnosis and delayed treatment of pneumomediastinum can lead to tension pneumothorax and ultimately to death (87), these patients must be monitored for at least 48 hours.
In a retrospective review of 62 consecutive adult patients diagnosed with SPM during an 11-year period (53), SPM was associated with a relatively benign condition, however, pneumothorax was seen in 32% of cases, a higher frequency than it was previously reported (36,37,45). This was probably due to the older age of the SPM patients in this study or due to the higher prevalence of preexisting lung diseases, particularly interstitial lung disease and bronchiolitis obliterans syndrome which according to severity appear to influence the clinical course of SP patients. In other studies of 136 (88) and 55 (50) pneumomediastinum patients as a result of blunt chest trauma, pneumothorax was seen in 68% and 84% respectively with limited or no mediastinal organ injury. Furthermore, Banki et al. in their study on pneumomediastinum patients (48) resulted in a negative association between pneumothorax and esophageal injury. This may be explained by the fact that when pneumothorax was present (78% of patients), the pneumomediastinum was more likely caused by a ruptured bleb than by mediastinal organ injury.
SP, pneumomediastinum, pneumopericardium, and epidural emphysema are extremely rare to occur simultaneously, especially when there is no history of trauma, underlying pathology or history of asthma. However, a young male who was referred to the emergency department by his primary care physician to rule out meningitis appeared with these findings without any preexisting condition or trauma (89).
In another case, the chest radiograph and the thoracic CT of an asthmatic 39-year-old male patient, who had admitted to the emergency room with respiratory distress and chest pain, revealed bilateral pneumothorax (90). A pneumomediastinum was also observed around the heart, together with subcutaneous emphysema. Subcutaneous emphysema can occur as the air passes below the skin and flows towards the neck and the face (91). In this patient the subcutaneous emphysema was specifically manifested in the neck. The treatment involved tube thoracostomy and chemical pleurodesis using talcum powder.
Recently, several cases have reported the concurrence of acute asthma attacks and pneumomediastinum or even emphysema (92-97) (Table 1). The first report of pneumomediastinum as an acute complication of occupational asthma was reported in a case of a 24-year-old baker as a consequence of a workplace exposure (99). It is not clear how to differentiate non-asthmatic from asthmatic pneumomediastinum patients by historical or demographic features, however, the co-existence of cough and chest pain may be a very useful prognostic factor for asthma related pneumomediastinum patients, especially for those experiencing wheezing episodes (95).
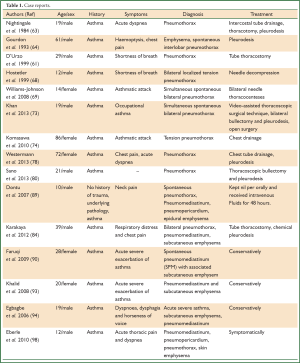
Full table
According to several reports asthma includes the most common etiology for pneumomediastinum (81), SP and pneumothorax with pneumomediastinum in children (100). SPM in children has been categorized as underdiagnosed (101). Dekel et al. during an investigation of the clinical characteristics and natural history of nine children with SPM identified three clinical patterns concerning long term sequelae of SP patients: patients without any long-term sequelae, patients with a tendency to airway hyperreactivity and subclinical asthma, and patients in whom SPM was the presenting feature of their asthma (102).
A study by Chiu et al. concerning sixteen cases of SPM, attempted to seek potential curable causes of SPM in children in order to minimize respiratory morbidity. Due to the high prevalence of bronchial asthma or cough (or both) in these patients, the researchers focused on the importance of the recent respiratory obstruction in the pathogenesis of SPM. Therefore, they suggested that diagnostic pulmonary function tests should be performed after acute episodes as well as designed to establish whether children with idiopathic SPM have asthma.
Recently, Wong et al. analyzed 87 pediatric patients concluding that patients younger than six years presented with secondary SPM should be vigilantly examined for predisposing causes. In adolescent patients with SPM without catastrophic events, asthma with exacerbation should be considered primary, whereas, extensive or invasive diagnostic examinations were not needed (103).
Association between SPM, pneumopericardium, pneumothorax and skin emphysema is extremely rare in children and young adults. A case of a 12-year-old boy with a bronchial asthma history presented with acute thoracic pain and dyspnea after sports, represents one of these cases. He was diagnosed with acute SPM in association with pneumopericardium, pneumothorax and skin emphysema, all of which were treated symptomatically (98).
Although the concurrence of bilateral pneumothorax with pneumomediastinum can rarely occur, it can be fatal during a serious asthma attack. Therefore, regarding the symptoms of chest pain and dyspnea, pneumomediastinum must always be considered in diagnosis. The faster the diagnosis and earlier the treatment are, the greater the chances for survival.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Guidelines: GINA Report GSfAMaP. Global Initiative for Asthma (GINA). 2012.
- Tamari M, Tomita K, Hirota T. Genome-wide association studies of asthma. Allergol Int 2011;60:247-52. [PubMed]
- Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59:469-78. [PubMed]
- He XY, Simpson JL, Wang F. Inflammatory phenotypes in stable and acute childhood asthma. Paediatr Respir Rev 2011;12:165-9. [PubMed]
- Bossley CJ, Fleming L, Gupta A, et al. Pediatric severe asthma is characterized by eosinophilia and remodeling without T(H)2 cytokines. J Allergy Clin Immunol 2012;129:974-82.e13.
- Krishnan JA, Lemanske RF Jr, Canino GJ, et al. Asthma outcomes: symptoms. J Allergy Clin Immunol 2012;129:S124-35. [PubMed]
- Killeen K, Skora E. Pathophysiology, diagnosis, and clinical assessment of asthma in the adult. Nurs Clin North Am 2013;48:11-23. [PubMed]
- Gergen PJ, Mullally DI, Evans R 3rd. National survey of prevalence of asthma among children in the United States, 1976 to 1980. Pediatrics 1988;81:1-7. [PubMed]
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012;18:716-25. [PubMed]
- Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy 2012;42:650-8. [PubMed]
- Noppen M, De Keukeleire T. Pneumothorax. Respiration 2008;76:121-7. [PubMed]
- Baumann MH, Noppen M. Pneumothorax. Respirology 2004;9:157-64. [PubMed]
- Laennec RT. A Trearise 011 Diseases 0/ rlre Clresr. (trans. by J. Fnrhes). New York: Hofner., 1819.
- Burke GJ. Pneumothorax complicating acute asthma. S Afr Med J 1979;55:508-10. [PubMed]
- Henry M, Arnold T, Harvey J. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003;58 Suppl 2:ii39-52. [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [PubMed]
- Leigh-Smith S, Harris T. Tension pneumothorax--time for a re-think? Emerg Med J 2005;22:8-16. [PubMed]
- Gupta D, Hansell A, Nichols T, et al. Epidemiology of pneumothorax in England. Thorax 2000;55:666-71. [PubMed]
- Sayar A, Turna A, Metin M, et al. Simultaneous bilateral spontaneous pneumothorax report of 12 cases and review of the literature. Acta Chir Belg 2004;104:572-6. [PubMed]
- Tschopp JM, Rami-Porta R, Noppen M, et al. Management of spontaneous pneumothorax: state of the art. Eur Respir J 2006;28:637-50. [PubMed]
- Noppen M. Spontaneous pneumothorax: epidemiology, pathophysiology and cause. Eur Respir Rev 2010;19:217-9. [PubMed]
- Wittich GR, Kusnick CA, Starnes VA, et al. Communication between the two pleural cavities after major cardiothoracic surgery: relevance to percutaneous intervention. Radiology 1992;184:461-2. [PubMed]
- Jantz MA, Antony VB. Pathophysiology of the pleura. Respiration 2008;75:121-33. [PubMed]
- Noppen M, Alexander P, Driesen P, et al. Manual aspiration versus chest tube drainage in first episodes of primary spontaneous pneumothorax: a multicenter, prospective, randomized pilot study. Am J Respir Crit Care Med 2002;165:1240-4. [PubMed]
- Sadikot RT, Greene T, Meadows K, et al. Recurrence of primary spontaneous pneumothorax. Thorax 1997;52:805-9. [PubMed]
- Andrivet P, Djedaini K, Teboul JL, et al. Spontaneous pneumothorax. Comparison of thoracic drainage vs immediate or delayed needle aspiration. Chest 1995;108:335-9. [PubMed]
- O’Rourke JP, Yee ES. Civilian spontaneous pneumothorax. Treatment options and long-term results. Chest 1989;96:1302-6. [PubMed]
- Inderbitzi R. Surgical thoracoscopy: report of experiences in Switzerland. Helv Chir Acta 1993;59:937-45. [PubMed]
- ohannesma PC, Lammers JW, van Moorselaar RJ, et al. Spontaneous pneumothorax as the first manifestation of a hereditary condition with an increased renal cancer risk. Ned Tijdschr Geneeskd 2009;153:A581.
- Yamaguchi M, Shiota T, Kobashi Y. Erdheim-Chester disease presenting with pneumothorax. Respiration 2011;82:552-6. [PubMed]
- Abbas A, Jones H, Kingston GT, et al. Malignant peripheral nerve sheath tumour presenting as a pneumothorax. Br J Radiol 2011;84:e197-9. [PubMed]
- Belchis DA, Shekitka K, Gocke CD. A unique, histopathologic lesion in a subset of patients with spontaneous pneumothorax. Arch Pathol Lab Med 2012;136:1522-7. [PubMed]
- Macklin CC. Transport of air along sheaths of pulmonic blood vessels from alveoli to mediastinum: Clinical implications. Arch Intern Med 1939;64:913-26.
- Ito S, Takada Y, Tanaka A, et al. A case of spontaneous pneumomediastinum in a trombonist. Kokyu To Junkan 1989;37:1359-62. [PubMed]
- MacLeod JB, Tibbs BM, Freiberger DJ, et al. Pneumomediastinum in the injured patient: inconsequential or predictive? Am Surg 2009;75:375-7. [PubMed]
- Caceres M, Ali SZ, Braud R, et al. Spontaneous pneumomediastinum: a comparative study and review of the literature. Ann Thorac Surg 2008;86:962-6. [PubMed]
- Mondello B, Pavia R, Ruggeri P, et al. Spontaneous pneumomediastinum: experience in 18 adult patients. Lung 2007;185:9-14. [PubMed]
- Weissberg D. Spontaneous mediastinal emphysema. Eur J Cardiothorac Surg 2004;26:885-8. [PubMed]
- Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg 2007;31:1110-4. [PubMed]
- Campillo-Soto A, Coll-Salinas A, Soria-Aledo V, et al. Spontaneous pneumomediastinum: descriptive study of our experience with 36 cases. Arch Bronconeumol 2005;41:528-31. [PubMed]
- Kaneki T, Kubo K, Kawashima A, et al. Spontaneous pneumomediastinum in 33 patients: yield of chest computed tomography for the diagnosis of the mild type. Respiration 2000;67:408-11. [PubMed]
- Abolnik I, Lossos IS, Breuer R. Spontaneous pneumomediastinum. A report of 25 cases. Chest 1991;100:93-5. [PubMed]
- Russo A, Del Vecchio C, Zaottini A, et al. Role of emergency thoracic ultrasonography in spontaneous pneumomediastinum. Two case report. G Chir 2012;33:285-96. [PubMed]
- Karakaya Z, Demir S, Sagay SS, et al. Bilateral spontaneous pneumothorax, pneumomediastinum, and subcutaneous emphysema: rare and fatal complications of asthma. Case Rep Emerg Med 2012;2012:242579.
- Newcomb AE, Clarke CP. Spontaneous pneumomediastinum: a benign curiosity or a significant problem? Chest 2005;128:3298-302. [PubMed]
- Patel A, Kesler B, Wise RA. Persistent pneumomediastinum in interstitial fibrosis associated with rheumatoid arthritis: treatment with high-concentration oxygen. Chest 2000;117:1809-13. [PubMed]
- Franquet T, Gimenez A, Torrubia S, et al. Spontaneous pneumothorax and pneumomediastinum in IPF. Eur Radiol 2000;10:108-13. [PubMed]
- Banki F, Estrera AL, Harrison RG, et al. Pneumomediastinum: etiology and a guide to diagnosis and treatment. Am J Surg 2013;206:1001-6; discussion 6. [PubMed]
- Bejvan SM, Godwin JD. Pneumomediastinum: old signs and new signs. AJR Am J Roentgenol 1996;166:1041-8. [PubMed]
- Molena D, Burr N, Zucchiatti A, et al. The incidence and clinical significance of pneumomediastinum found on computed tomography scan in blunt trauma patients. Am Surg 2009;75:1081-3. [PubMed]
- Rezende-Neto JB, Hoffmann J, Al Mahroos M, et al. Occult pneumomediastinum in blunt chest trauma: clinical significance. Injury 2010;41:40-3. [PubMed]
- Ball CG, Kirkpatrick AW, Laupland KB, et al. Incidence, risk factors, and outcomes for occult pneumothoraces in victims of major trauma. J Trauma 2005;59:917-24; discussion 924-5. [PubMed]
- Iyer VN, Joshi AY, Ryu JH. Spontaneous pneumomediastinum: analysis of 62 consecutive adult patients. Mayo Clin Proc 2009;84:417-21. [PubMed]
- Cicak B, Verona E, Mihatov-Stefanovic I, et al. Spontaneous pneumomediastinum in a healthy adolescent. Acta Clin Croat 2009;48:461-7. [PubMed]
- Lee YJ, Jin SW, Jang SH, et al. A case of spontaneous pneumomediastinum and pneumopericardium in a young adult. Korean J Intern Med 2001;16:205-9. [PubMed]
- Gabor SE, Renner H, Maier A, et al. Tension pneumomediastinum after severe vomiting in a 21-year-old female. Eur J Cardiothorac Surg 2005;28:502-3. [PubMed]
- Yellin A, Lidji M, Lieberman Y. Recurrent spontaneous pneumomediastinum. The first reported case. Chest 1983;83:935. [PubMed]
- Natale C, D’Journo XB, Duconseil P, et al. Recurrent spontaneous pneumomediastinum in an adult. Eur J Cardiothorac Surg 2012;41:1199-201. [PubMed]
- Tanaka F, Itoh M, Esaki H, et al. Secondary spontaneous pneumothorax. Ann Thorac Surg 1993;55:372-6. [PubMed]
- Baumann MH. Management of spontaneous pneumothorax. Clin Chest Med 2006;27:369-81. [PubMed]
- D’Urzo AD, D’Urzo DK, Chapman KR. Case report: pneumothorax and asthma. Can Fam Physician 1999;45:1524-5. [PubMed]
- Light RW. Management of spontaneous pneumothorax. Am Rev Respir Dis 1993;148:245-8. [PubMed]
- Nightingale RC, Flower CD. Encysted pneumothorax, a complication of asthma. Br J Dis Chest 1984;78:98-100. [PubMed]
- Gourdon C, Dietemann A, Beigelman C, et al. Recurrent interlobar pneumothorax in an asthmatic patient. Eur Respir J 1993;6:748-9. [PubMed]
- Bildstein F, Dalphin JC, Clement F, et al. Interlobar pneumothorax. Rev Mal Respir 1988;5:407-8. [PubMed]
- Rabinowitz JG, Kongtawng T. Loculated interlobar air-fluid collection in congestive heart failure. Chest 1978;74:681-3. [PubMed]
- Vincent M, Tourvielle O, Beguier M, et al. Interlobar pneumothorax. Rev Pneumol Clin 1984;40:7-11. [PubMed]
- Hostetler MA, Davis CO. Bilateral localized tension pneumothoraces refractory to needle decompression. Pediatr Emerg Care 1999;15:322-4. [PubMed]
- Williams-Johnson J, Williams EW, Hart N, et al. Simultaneous spontaneous bilateral pneumothoraces in an asthmatic. West Indian Med J 2008;57:508-10. [PubMed]
- Hartin DJ, Kendall R, Boyle AA, et al. Case of the month: Buffalo chest: a case of bilateral pneumothoraces due to pleuropleural communication. Emerg Med J 2006;23:483-6. [PubMed]
- Schorlemmer GR, Khouri RK, Murray GF, et al. Bilateral pneumothoraces secondary to latrogenic buffalo chest. An unusual complication of median sternotomy and subclavian vein catheterization. Ann Surg 1984;199:372-4. [PubMed]
- Flume PA. Pneumothorax in cystic fibrosis. Chest 2003;123:217-21. [PubMed]
- Khan WA, Curl-Roper T, Helm J, et al. Simultaneous bilateral spontaneous pneumothoraces: a case of occupational asthma. BMJ Case Rep 2013;2013. pii: bcr2013010057.
- Komasawa N, Ueki R, Kusuyama K, et al. Case of tension pneumothorax associated with asthma attack during general anesthesia. Masui 2010;59:614-7. [PubMed]
- Kang SW, Bach JR. Maximum insufflation capacity. Chest 2000;118:61-5. [PubMed]
- Dwight P, Poenaru D. Duodenal perforation associated with breath stacking and annular pancreas. J Pediatr Surg 2004;39:1593-4. [PubMed]
- Suri P, Burns SP, Bach JR. Pneumothorax associated with mechanical insufflation-exsufflation and related factors. Am J Phys Med Rehabil 2008;87:951-5. [PubMed]
- Westermann EJ, Jans M, Gaytant MA, et al. Pneumothorax as a complication of lung volume recruitment. J Bras Pneumol 2013;39:382-6. [PubMed]
- Gosein MA, Ameeral A, Konduru SK, et al. Tuberous sclerosis presenting with spontaneous pneumothorax secondary to lymphangioleiomyomatosis; previously mistaken for asthma. BMJ Case Rep 2013;2013. pii: bcr2013009969.
- Sano A, Fukami T, Murakawa T, et al. Recurrent Pneumothorax Related to Swyer-James Syndrome. Ann Thorac Cardiovasc Surg 2013. [Epub ahead of print]. [PubMed]
- Inoue M, Nakagawa K, Kameda M, et al. Video-assisted thoracoscopic bullectomy for spontaneous pneumothorax in a Swyer-James syndrome patient. Jpn J Thorac Cardiovasc Surg 2002;50:439-42. [PubMed]
- Soni R, Barnes D. Macleod’s syndrome presenting with spontaneous pneumothorax. Respirology 1999;4:275-7. [PubMed]
- Guidelines BTSaSI, Network Bgotm, asthma. o. Revised April 2004. Available online: http://www.britthoracic. org.uk/c2/uploads/asthmafull.pdf (accessed11 august 2005).
- Ludwig J, Kienzle GD. Pneumothorax in a large autopsy population. A study of 77 cases. Am J Clin Pathol 1978;70:24-6. [PubMed]
- Saltet JF. Pneumothorax. Lancet 1979;1:671. [PubMed]
- Maunder RJ, Pierson DJ, Hudson LD. Subcutaneous and mediastinal emphysema. Pathophysiology, diagnosis, and management. Arch Intern Med 1984;144:1447-53. [PubMed]
- Rim T, Bae JS, Yuk YS. Life-Threatening Simultaneous Bilateral Spontaneous Tension Pneumothorax - A case report. Korean J Thorac Cardiovasc Surg 2011;44:253-6. [PubMed]
- Dissanaike S, Shalhub S, Jurkovich GJ. The evaluation of pneumomediastinum in blunt trauma patients. J Trauma 2008;65:1340-5. [PubMed]
- Dontu VS, Kramer D. Spontaneous pneumothorax, pneumomediastinum, and epidural emphysema presenting as neck pain suspicious for meningitis. Pediatr Emerg Care 2007;23:469-71. [PubMed]
- Karakaya Z, Demir S, Sagay SS, et al. Bilateral spontaneous pneumothorax, pneumomediastinum, and subcutaneous emphysema: rare and fatal complications of asthma. Case Rep Emerg Med 2012;2012:242579.
- Maravelli AJ, Skiendzielewski JJ, Snover W. Pneumomediastinum acquired by glass blowing. J Emerg Med 2000;19:145-7. [PubMed]
- Faruqi S, Varma R, Greenstone MA, et al. Spontaneous pneumomediastinum: a rare complication of bronchial asthma. J Asthma 2009;46:969-71. [PubMed]
- Khalid MS, Ahmad N, Moin S, et al. Spontaneous pneumomediastinum: a rare complication of acute asthma. Ir J Med Sci 2008;177:393-6. [PubMed]
- Egbagbe EE, Elusoji SO. Pneumomediastinum and subcutaneous emphysema associated with asthma exacerbation. J Pak Med Assoc 2006;56:287-9. [PubMed]
- Chiu CY, Wong KS, Yao TC, et al. Asthmatic versus non-asthmatic spontaneous pneumomediastinum in children. Asian Pac J Allergy Immunol 2005;23:19-22. [PubMed]
- Bedolla-Barajas M, Hernandez-Colin DD, Miramontes-Luna E, et al. Spontaneus pneumomediastinum associated with exacerbation of asthma during the epidemic of influenza A H1N1: Inform of four cases. Rev Alerg Mex 2011;58:142-6. [PubMed]
- Hashim T, Chaudry AH, Ahmad K, et al. Pneumomediastinum from a severe asthma attack. JAAPA 2013;26:29-32. [PubMed]
- Eberle C, Junger K, Debatin KM, et al. Spontaneously occurring pneumomediastinum related to a pneumopericardium, a pneumothorax and a skin emphysema in a 12-year old boy. Klin Padiatr 2010;222:40-4. [PubMed]
- Pala G, Pignatti P, Moscato G. Pneumomediastinum is a possible acute complication of severe occupational asthma. Iran J Allergy Asthma Immunol 2012;11:79-81. [PubMed]
- Chalumeau M, Le Clainche L, Sayeg N, et al. Spontaneous pneumomediastinum in children. Pediatr Pulmonol 2001;31:67-75. [PubMed]
- Versteegh FG, Broeders IA. Spontaneous pneumomediastinum in children. Eur J Pediatr 1991;150:304-7. [PubMed]
- Dekel B, Paret G, Szeinberg A, et al. Spontaneous pneumomediastinum in children: clinical and natural history. Eur J Pediatr 1996;155:695-7. [PubMed]
- Wong KS, Wu HM, Lai SH, et al. Spontaneous pneumomediastinum: analysis of 87 pediatric patients. Pediatr Emerg Care 2013;29:988-91. [PubMed]


