Prognostic value of lymph node ratio after induction therapy in stage IIIA/N2 non-small cell lung cancer: a monocentric clinical study
Introduction
Lung cancer remains the leading cause of cancer related death all over the world (1). Nearly 30% of newly diagnosed patients with non-small cell lung cancer (NSCLC) suffer from disease with mediastinal lymph node involvement (2). Best treatment modalities for these patients remain a matter of controversy, particularly concerning the indications for surgery. Nevertheless, prospective randomized trials have shown a survival benefit for selected patients with stage IIIA/B treated by three-modality-treatment including surgery (3,4).
To provide best outcomes and to avoid unnecessary surgery in patients that would not profit from tumor resection, patient selection is a crucial task for the treating oncologic surgeon. In patients with mediastinal lymph node involvement who lack distant metastasis and are fit to undergo resection usually two cycles of platinum-based neoadjuvant chemotherapy are applied. After induction treatment patients are usually reassessed for further treatment modalities. Whilst some authors suggest restaging via mediastinoscopy or endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA), in the guidelines by the National Comprehensive Cancer Network (NCCN) indication for surgery is based solely on apparent radiological progression under induction therapy (5). Interestingly, after resection even in these highly selected patients long term survival is highly variable and although mediastinal downstaging has been shown to be the most robust prognosticator in this patient collective, recent data suggests that in the subgroup with persistent mediastinal lymph node metastasis after induction therapy, there are patients with a favorable prognosis (6,7). The lymph node ratio (LNR), which describes the number of involved lymph nodes divided by the number of resected lymph nodes has been suggested as one tool to identify these patients (8-11).
We hypothesized that in case of a persisting mediastinal lymph node involvement for stage IIIA/N2 NSCLC after induction therapy a low LNR could identify patients with a favorable prognosis.
Methods
The study was approved by our local ethics committee (AZ 127/17) and registered as a clinical trial (DRKS00013150). We retrospectively analyzed the data of 78 patients with histologically proven NSCLC stage IIIA/N2 that underwent neoadjuvant treatment with intended surgery between May 2009 and February 2016. Initial staging was performed by FDG-PET-CT and cerebral MRI in addition to the chest computed tomography (CT). Mediastinal staging was performed in case of suspicion of mediastinal lymph node metastasis in PET-scan by EBUS-TBNA, which in case of a negative result was followed by a mediastinoscopy. In some cases, mediastinal lymph node involvement was histologically proven by thoracoscopy (e.g., lymph node stations 5, 6). Treatment modalities were determined in the institutions multidisciplinary tumor board (MDT). Induction therapy consisted of a standardized regimen of two cycles of a platinum-based chemotherapy. After completion of the neoadjuvant protocol a chest CT-scan was performed for restaging. If resectability was given, patients were planned for operation without repeated invasive mediastinal staging. Adequate anatomic resection was performed including systematic lymphadenectomy in accordance with the ESTS guidelines (12).
Tissue specimen were investigated by the Institute for Surgical Pathology the Medical Center-University of Freiburg. Absence of tumor in the lymph nodes was considered as complete mediastinal downstaging (ypN0). Presence of tumor in hilar lymph nodes only was considered as partial mediastinal downstaging (ypN1), all other cases as persisting mediastinal involvement (ypN2). Involvement of one mediastinal lymph node station was seen as single level, lymph node metastasis in 2 or more different mediastinal lymph node stations was considered a multilevel N2 status. Overall survival (OS) was calculated from surgery until death or last follow up, disease free survival (DFS) from surgery until disease relapse or death respectively. Postoperative mortalities within 30 days after surgery were excluded from the analysis.
Adjuvant treatment was discussed in the MDT. Adjuvant radiotherapy was recommended for all patients with persistent mediastinal lymph node metastasis; in 6 cases adjuvant chemo- and radiotherapy was applied because of good response to neoadjuvant chemotherapy and persistent mediastinal lymph node metastasis.
Statistical Analyses were performed by SPSS Version 23. The prognostic influence of variables on survival or disease free interval was assessed using log-rank tests and Cox proportional hazard models. OS was defined as the time interval from surgery to death by any cause. Disease-free survival (DFS) was defined as the time interval from surgery to tumor recurrence or death by any cause. Data sets were tested for normality using the D’Agostino-Pearson omnibus normality test. In normally distributed data-sets Student’s t-test, in non-normally distributed data-sets Mann-Whitney test was performed. Categorical variables were tested for dependency using Fisher’s exact test and the odds-ratio (OR) as well as the associated confidence intervals were calculated.
All tests were considered significant for P values below 0.05. A P value between 0.05–0.1 was considered a statistical trend.
Results
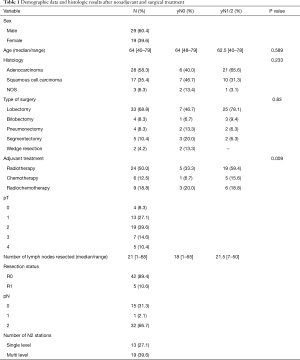
The cohort included 29 (60.4%) males and 19 (39.6%) females with a median age of 64 (range, 40–79). A lobectomy was performed in 33 (68.8%), bilobectomy and pneumonectomy in 4 (8.3%) cases respectively. In 5 (10.4%) patients a segmentectomy and twice (4.2%) a wedge resection was performed. Sublobar resections were performed in case of limited pulmonary reserve. Histologic findings revealed adenocarcinoma in 28 (58.3%), squamous cell carcinoma in 17 (35.4%) patients and 3 (6.3%) NSCLC that were not attributable. Median number of resected lymph nodes was 21.5 (range, 7–50) and median number of lymph node metastases was 5.5 (range, 1–20); complete mediastinal downstaging (N2→pN0) was observed in 15 (31.3%) patients and in 1 (2.1%) patient down staging to pN1 after induction therapy occurred. In case of persisting mediastinal lymph node involvement in 14 (43.8%) patients one level, in 11 (34.3%) two levels and in 7 (21.9%) ≥3 levels were involved. Defining the cut off for the LNR at ≤0.33 there were 40 patients with a low LNR and 7 patients with LNR >0.33.
This cut off was selected considering the preceding study by Renaud et al. which included the same patient collective after induction therapy. Other Cut off points that were reported in the literature (LNR 0.14, LNR 0.22 and LNR 0.25) were calculated as well.
Adjuvant radiotherapy was performed in 24 (50.0%) cases, 6 (12.5%) patients received adjuvant chemotherapy and 9 (18.8%) adjuvant radio- and chemotherapy. Patient characteristics are summarized in Table 1.
Full table
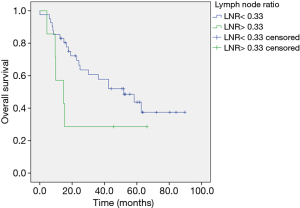
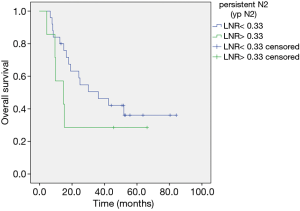
Median follow up was 30.1 months (range, 4.4–89.7). In univariate analysis mediastinal down staging was associated with a longer OS without reaching significance: 52.2 (range, 5.9–89.7) months for ypN0 versus 24.6 (4.4–84.2) for ypN1/2 (HR, 2.76; 95% CI, 0.86–5.42, P=0.0973). In multivariate analysis adjusted for age absence of lymph node metastasis after neoadjuvant chemotherapy was identified as a favorable prognostic marker (HR, 2.76; 95% CI, 1.07–7.1, P=0.0348) (Figure 1). LNR ≤0.33 was linked to a better OS of 39.3 (range, 5.9–89.7) months compared to LNR >0.33 (14.7 months, range, 4.4–66.2 months) in univariate analysis applying the log rank test (P=0.037) (Figure 2). In multivariate analysis adjusted for age and T-status this continued to be a statistical trend (HR, 2.82; 95% CI, 0.98–8.14, P=0.1. In patients with persistent lymph node involvement there was a better OS for patients with low LNR compared to a high LNR, however not statistically significant (30.1, range, 6.6–84.2 months versus 14.7, range, 4.4–66.2 months for LNR >0.33, P=0.145) (Figure 3).
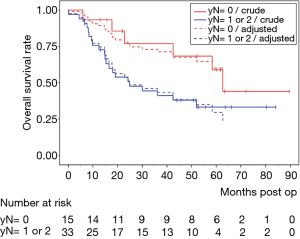
Median LNR was 0.14 and was chosen as further cut off point. A low LNR ≤0.14 showed no influence on OS (30.6, range, 5.9–89.7 months vs. 30.1, range, 4.4–80.4 months for LNR >0.14). Other authors reported on LNR cut off at 0.22 that was linked to a longer OS in our study (39.4, range, 5.9–89.7 months for LNR ≤0.22 vs. 16.5, range, 4.4–80.4 months for LNR >0.22, P=0.162) as well as 0.25 (33.2, range, 5.9–89.7 months for LNR ≤0.25 vs. 15.3, range, 4.4–80.4 months for LNR >0.25, P=0.348) but without reaching statistical significance.
There was no difference in OS between single level or multilevel mediastinal involvement (33.1, range, 5.9–89.7 months for single level N2 versus 24.2, range, 4.4–84.2 months for multilevel N2, P=0.318).
Median DFS was 36.2 months (range, 4.4–89.7). A longer DFS was observed for patients with mediastinal down staging (52.2, range, 5.6–89.7 months) compared to persistent N2 (27.5, range, 4.4–84.2 months) without reaching significance in univariate and multivariate analysis (HR, 2.18; 95% CI, 0.86–5.53, P=0.1). In patients with a LNR ≤0.33 a median DFS of 37.8 (range, 5.6–89.7) months and in case of a LNR >0.33 a median DFI of 15.3 (range, 4.4–82.1) months was observed. DFS for patients with single level mediastinal involvement was 33.1 (range, 5.6–89.7) months; for multilevel N2 a DFS of 39.3 (range, 4.4–84.2) months could be observed. In univariate and multivariate analysis no statistical differences were revealed in these subgroups (HR, 2.54; 95% CI, 0.94–6.83, P=0.17).
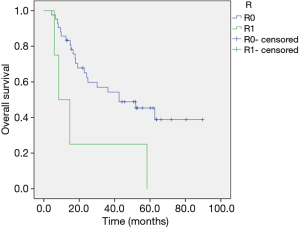
OS was strongly influenced by resection status as median survival for a complete resection was 33.2 (range, 4.4–89.7) months compared to 14.7 (range, 5.9–84.2) months in case of R1 or Rx (P=0.041) (Figure 4).
Discussion
The best treatment modalities for stage IIIA N2 NSCLC are still a matter of debate. It is now widely accepted that in selected patients, surgery is an important part of multimodal therapy, resulting in 5 year survival rates of 30–44% (3,4,7). Nevertheless, this patient group is highly heterogeneous and finding the right treatment modalities for the variable tumor biology in this tumor stage is crucial.
The most commonly applied and best studied prognostic factor after induction chemotherapy is mediastinal downstaging. Going in line with previous studies we show that complete absence of lymph node metastasis after induction therapy is associated with the best outcome reaching an OS of 52.2 months for patients with ypN0 after neoadjuvant treatment in opposition to 24.6 months in case of persisting ypN2. A major drawback for using mediastinal downstaging as a prognosticator to aid in treatment decision making is the difficulty to reliably rule out presence or absence of remaining lymph node metastasis previous to surgery. Sensitivity of PET-CT to detect mediastinal lymph node metastasis is around 74% for lymph nodes of small size (13). After induction therapy accuracy remains comparable, correctly identifying persistent lymph nodes in about 70% and T-stage in about half of the cases, hence rendering PET-scans an inadequate tool for restaging. Other authors performed mediastinoscopy or EBUS-TBNA, the latter with an acceptable sensitivity of 82% and a negative predictive value of 88%, to exclude a persisting mediastinal lymph node involvement and to consequently exclude patients from surgery in case of mediastinal N+ (14-16). However, a reliable statement about absence of mediastinal lymph node metastasis can only be made after surgery including systematic mediastinal lymphadenectomy (17,18). Therefore, going along with the NCCN-guidelines, at our institution a restaging chest-CT is carried out after neoadjuvant treatment to confirm persistent resectability and to offer surgery to all patients in whom an R0 situation is achievable (5).
Interestingly, in the subgroup of patients with persistent lymph node metastasis after induction therapy some patients show a favorable prognosis. To identify these cases and further differentiate the mediastinal lymph node involvement other prognostic factors were introduced in recent literature. Some authors argued that the number of involved metastatic lymph nodes might be a good prognostic factor (19,20). However, the number of positive lymph nodes depend highly on the dissection technique (sampling versus complete lymphadenectomy), fragmentation and histopathologic count and is greatly influenced by these factors. To make these observations more robust against the described confounders the LNR was suggested as a prognosticator (8-11). As it is a relative value, the LNR is claimed to be less affected by lymph node fragmentation or dissection technique, because if nodes are fragmented the number of metastatic lymph nodes and resected lymph nodes rise equally.
In our study we could show a better survival in patients with a low LNR after induction therapy. Different cutoffs were applied, however the best stratification was reached when the cut off was set at 0.33, as has been described for patients with stage IIIA/N2 before (16). Different other cutoffs for LNR have been described in the literature: Matsugama et al. reported a worse OS in case of an LNR >12% and Jonalgadda et al. divided their population into three groups (<15%, between 15 and 50% and >50%) and observed worse survival with increasing LNR. Furthermore, Nwogu and colleagues also created three groups (<24%, 25–49% and 50% and more) and showed the same impact on survival. To date it remains unclear which cutoff for LNR best predicts the outcome in this patient collective.
Furthermore, in the subgroup with persistent lymph node metastasis, patients with a lower LNR showed a favorable prognosis. This finding goes in line with the only previously conducted study on this matter by Renaud and colleagues. However, studies are not completely comparable, as the former authors excluded patients with preoperatively persisting N2 after induction therapy (16). These patients actually compose another subgroup with occult lymph node metastasis after induction therapy, which have an a priori more favorable prognosis (17).
The study at hand is limited in its statistical power due to the small sample size, monocentric setting and the retrospective design especially concerning the analysis of the subgroup with persistent lymph node metastasis. Even in a high-volume center only a few patients qualify for this kind of multimodal treatment. Nevertheless, we believe that information on this important topic is scarce and we add important data.
In conclusion, to date complete mediastinal downstaging remains the best prognosticator for stage IIIA/N2 NSCL after induction therapy. Additionally, the LNR could aid in identifying patients with persisting mediastinal lymph node involvement who can achieve long term survival and thus aid in therapeutic decision making. It has to be acknowledged, that the prognostic markers that are currently applied are insufficient and future studies should focus on robust and reproducible molecular markers which can identify distinctive tumor biologies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our local ethics committee (AZ 127/17) and registered as a clinical trial (DRKS00013150).
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Johnson DH, Rusch VW, Turrisi AT. Scalpels, beams, drugs, and dreams: challenges of stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:415-8. [Crossref] [PubMed]
- Eberhardt WE, Pottgen C, Gauler TC, et al. Phase III Study of Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients With Resectable Stage IIIA(N2) and Selected IIIB Non-Small-Cell Lung Cancer After Induction Chemotherapy and Concurrent Chemoradiotherapy (ESPATUE). J Clin Oncol 2015;33:4194-201. [Crossref] [PubMed]
- Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 1995;13:1880-92. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Dooms C, Verbeken E, Stroobants S, et al. Prognostic stratification of stage IIIA-N2 non-small-cell lung cancer after induction chemotherapy: a model based on the combination of morphometric-pathologic response in mediastinal nodes and primary tumor response on serial 18-fluoro-2-deoxy-glucose positron emission tomography. J Clin Oncol 2008;26:1128-34. [Crossref] [PubMed]
- Decaluwe H, De Leyn P, Vansteenkiste J, et al. Surgical multimodality treatment for baseline resectable stage IIIA-N2 non-small cell lung cancer. Degree of mediastinal lymph node involvement and impact on survival. Eur J Cardiothorac Surg 2009;36:433-9. [Crossref] [PubMed]
- Jonnalagadda S, Arcinega J, Smith C, et al. Validation of the lymph node ratio as a prognostic factor in patients with N1 nonsmall cell lung cancer. Cancer 2011;117:4724-31. [Crossref] [PubMed]
- Nwogu CE, Groman A, Fahey D, et al. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann Thorac Surg 2012;93:1614-9; discussion 1619-20. [Crossref] [PubMed]
- Urban D, Bar J, Solomon B, et al. Lymph node ratio may predict the benefit of postoperative radiotherapy in non-small-cell lung cancer. J Thorac Oncol 2013;8:940-6. [Crossref] [PubMed]
- Wang CL, Li Y, Yue DS, et al. Value of the metastatic lymph node ratio for predicting the prognosis of non-small-cell lung cancer patients. World J Surg 2012;36:455-62. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:178s-201s.
- Cetinkaya E, Usluer O, Yilmaz A, et al. Is endobronchial ultrasound-guided transbronchial needle aspiration an effective diagnostic procedure in restaging of non-small cell lung cancer patients? Endosc Ultrasound 2017;6:162-7. [Crossref] [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. The efficacy of restaging endobronchial ultrasound in patients with non-small cell lung cancer after preoperative therapy. Ann Thorac Surg 2014;98:1008-12. [Crossref] [PubMed]
- Renaud S, Falcoz PE, Olland A, et al. Mediastinal downstaging after induction treatment is not a significant prognostic factor to select patients who would benefit from surgery: the clinical value of the lymph node ratio. Interact Cardiovasc Thorac Surg 2015;20:222-7. [Crossref] [PubMed]
- De Waele M, Serra-Mitjans M, Hendriks J, et al. Accuracy and survival of repeat mediastinoscopy after induction therapy for non-small cell lung cancer in a combined series of 104 patients. Eur J Cardiothorac Surg 2008;33:824-8. [Crossref] [PubMed]
- Cerfolio RJ, Maniscalco L, Bryant AS. The treatment of patients with stage IIIA non-small cell lung cancer from N2 disease: who returns to the surgical arena and who survives. Ann Thorac Surg 2008;86:912-20; discussion 912-20. [Crossref] [PubMed]
- Fukui T, Mori S, Yokoi K, et al. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol 2006;1:120-5. [Crossref] [PubMed]
- Haager B, Osei-Agyemang T, Passlick B, et al. Lung cancer: is surgery an option for persisting N2 after induction therapy? Zentralbl Chir 2015;140:99-103. [PubMed]