Non-intubated video-assisted thoracoscopic lung biopsy for interstitial lung disease: a single-center experience
Introduction
Surgical lung biopsy plays an important role in providing pathological findings in patients with interstitial lung disease (ILD) (1). A recent study of approximately 12,000 surgical lung biopsies from the Nationwide Inpatient Sample revealed that in-hospital mortality after surgery was 1.7% for elective procedures and 16.0% for non-elective procedures (2). The mortality and morbidity associated with surgical lung biopsy for ILD are not negligible (3,4). Acute exacerbation of ILD generally has a very poor prognosis (5), and acute exacerbation of ILD after biopsy is one of the major causes of mortality. The etiology of acute exacerbation of ILD remains unclear. The typical video-assisted thoracoscopic (VATS) procedure is performed under general anesthesia with positive mechanical ventilation, which is thought to trigger or worsen acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (6-9).
Non-intubated VATS is another option for lung biopsy in ILD. As the VATS technique and anesthetic technology have evolved, the use of non-intubated VATS has become widely accepted in thoracic surgery. Reports have described applications in wedge resection, segmentectomy, lobectomy, and bronchoplasty. And several studies reported safety and feasibility of non-intubated VATS biopsy in patients with undetermined ILD (10-12). Non-intubated VATS is known to have fewer postoperative complications and faster recovery times than typical VATS, because it does not use muscle relaxants, double lumen tube intubation, and injuries from positive pressure ventilation (13-15). Therefore, we hypothesized that non-intubated thoracic surgery would decrease postoperative complications. The aim of our study was to assess the feasibility and safety of non-intubated VATS lung biopsy.
Methods
Study population
This study was approved by the institutional review board. The need for individual patient consent was waived. We retrospectively analyzed ILD subjects undergoing VATS lung biopsy at a single institution between January 2016 and June 2016. The inclusion criteria were subjects aged >20 years with chest CT findings of ILD. Exclusion criteria included preoperative ICU hospitalization, preoperative ALI, hemodynamic instability, and redo thoracotomy. A total of 35 consecutive subjects were included for the analysis and divided into two groups—an intubated group (n=25) and a non-intubated group (n=10). The allocation of a patient to each group was determined according to the preference of each surgeon and the consent of the patient. And the exact diagnosis was not known before operation and confirmed by postoperative pathological results. Early outcome data including intraoperative and postoperative variables were compared between the groups.
Surgical procedure
Intubated group
VATS was performed in intubated subjects under general anesthesia. In the operating room, the standard monitoring devices including electrocardiography (EKG), pulse oximeter, end-tidal carbon dioxide (ETCO2) were applied. Endotracheal intubation was performed via the routine double-lumen endotracheal intubation procedure. An arterial catheter was placed in the radial artery opposite the surgical site for invasive arterial blood pressure monitoring. A 5.5- or 10.5-mm camera port and one 5.5-mm instrumentation port were inserted. A thorough assessment of the pleura and lung surface was made, and wedge resection was performed with an Endo GIA 60–4.8 mm endoscopic stapler (Covidien Endo GIA Universal Roticulator). At the end of the procedure, one of the chest tubes was left in situ.
Non-intubated group
For non-intubated VATS, though some other groups used intercostal blocks or only local anesthesia (11,12), our group used epidural anesthesia. Thoracic epidural catheter was inserted before operation in the pre-anesthesia room and the correct position of catheter was confirmed by test dose and/or fluoroscopy.
In the operating room, monitoring was the same with the intubated group. Four mL of 4% lidocaine nebulization (Nebulizer: PARI, Moosstrasse3, Germany) was applied for 15 minutes to prevent coughing before operation. While the patient was on lidocaine nebulization, the radial artery was cannulated and drug injection for thoracic blockade was started. Three to four mL of the local anesthetic [0.75% ropivacaine (Ropivacaine HCl, HANLIM PHARM)] was administered incrementally through epidural catheter. A total of 13–15 mL of 0.75% ropivacaine (mixed with 50 µg fentanyl) was administered. The final check of the sensory block before operation covered from T3 to T10 after 15 minutes.
Conscious sedation was maintained during operation with a remifentanil (0.0–0.5 mcg/kg/min) or dexmedetomidine (0.3–0.5 mcg/kg/hr) infusion and the dose was titrated to maintain a bispectral index (BIS) of 60–80 during surgery. Five L/min of 100% oxygen was administered via facial mask with ETCO2 monitor line attached to the mask during the operation. In case of low saturation (<95%), the O2 fraction of facial mask was increased and patient was encouraged to breathe deeply.
A laryngeal mask airway (LMA) and bronchial blocker were prepared in case of conversion to general anesthesia. We used the single incision laparoscopic surgery (SILS) for non-intubated VATS biopsy to decrease patient discomfort and achieve better control of collapse and inflation of the operated lung. This was also effective in reducing coughing by minimizing irritation of the pleura, which can be caused by airflow. The operation started with 4 cm of skin incision on the left 5th intercostal space after enough skin and subcutaneous tissue local infiltration. Then 4 cm uniport utility (Glove port 4220-AS-3, Nelis medical, Korea) was inserted and tissue biopsies were performed. At the time when the scope was inserted, the lung became atelectatic and if not, within first 10 minutes. The lung remained atelectatic until the end of the operation. An intraoperative vagal nerve block was not required because only simple wedge resection was performed.
In both groups, lung biopsy was performed by one or two wedge resections of lung regions deemed radiologically and macroscopically more representative for diagnosis using standard method. At the end of the procedure, one of the chest tubes was left in situ.
Postoperative management
All subjects were evaluated via chest radiography every day. Chest tube removal was performed if chest tube drainage was less than 200 mL for 24 hours with sufficient lung recruitment and no air leakage on chest radiography. And subjects were discharged the next day after a chest tube removal if there is no specific medical problem.
Follow-up
Early mortality was defined as mortality within the first 30 days after surgery. ALI was defined according to the following criteria occurring within 7 days after surgery that was unrelated to other documented causes of ALI; (I) acute onset; (II) bilateral infiltration with pulmonary edema; (III) a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) between 201 and 300 mmHg, regardless of the level of positive end-expiratory pressure. ARDS was included as subcategories of ALI and defined as more severe hypoxemia (PaO2/FiO2 ≤200 mmHg).
Statistical analysis
Analyses comparing patient parameters and outcomes by study group included the two sample t-test or Mann-Whitney U test for continuous variables, and the χ2 analysis or the Fisher exact test for categorical variables. A P value less than 0.05 was considered statistically significant. Statistical analysis was carried out using SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA).
Results
Subject characteristics
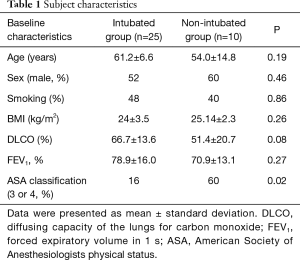
The procedures were successfully completed in all 35 subjects. The baseline characteristics of the two groups are summarized in Table 1. There were no statistically significant differences in age, sex, smoking status, body mass index (BMI), or forced expiratory volume in 1 second (FEV1) between the two groups, but the non-intubated group had a significantly higher American Society of Anesthesiologists physical status (ASA) score than that of the intubated group III or at of the intubated group non-intubated group had a significance. The mean preoperative carbon monoxide diffusing capacity (DLCO) of the non-intubated group was 51.4%, which was lower than that of the intubated group (66.7%; P=0.08).
Full table
Perioperative data
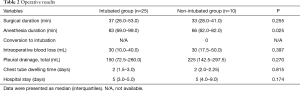
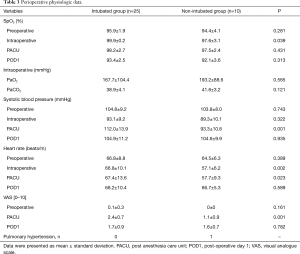
Perioperative data pertaining to the two groups are summarized in Tables 2 and 3. Comparisons between the intubated and non-intubated groups showed no significant differences in operation time, intraoperative blood loss, chest tube dwelling time, overall postoperative chest drainage volume, or hospital stay. However, duration of anesthesia was significantly lower in the non-intubated group (median, 66 min; interquartiles, 62–82) than in the intubated group (median, 83 min; interquartiles, 69–99; P=0.025). And the non-intubated group showed low level of intraoperative saturation and heart rate. Visual analogue scale, systolic blood pressure and heart rate at post anesthesia care unit were also lower in the non-intubated group.
Full table
Full table
Postoperative complications
No early mortality or significant complications, including postoperative ALI, prolonged air leak (≥5 days), postoperative bleeding, postoperative pneumonia (within ≤1 month), wound infection, or ICU admission after surgery, occurred in either group.
Histological results
We were able to obtain a final histological diagnosis in all subjects (diagnostic yield 100%). In the intubated group, unusual interstitial pneumonia (UIP, N=11, 44%) was the most common, followed by nonspecific interstitial pneumonia (NSIP, N=8, 32%), organizing pneumonia (N=4, 16%), and granulomatous ILD (N=1, 4%). In the non-intubated group, NSIP was the most common (N=8, 80%), with one subject diagnosed with UIP and one with desquamative interstitial pneumonia (DIP).
Discussion
As a small sized pilot study, we could not confirm whether non-intubated lung biopsy reduces acute exacerbation of ILD compared to normal VATS lung biopsy. However, this study demonstrated the safety and feasibility of non-intubated VATS lung biopsy in ILD subjects.
General anesthesia with double lumen tube intubation was previously considered essential in VATS. However, to reduce complications related to intubation or mechanical ventilation, non-intubated VATS is increasingly being performed in selected patients (16). Non-intubated surgery reduces intubation-related complications compared to conventional VATS surgery under general anesthesia. In addition, as muscle relaxants are not required and the amount of anesthetic drug used during the operation is lower, non-intubated surgery enables early ambulation after the operation and minimizes respiratory muscle weakness and digestive system dysfunction (17). Therefore, recovery is accelerated after non-intubated surgery. This reduces the duration and costs of hospital stays (18,19).
We applied non-intubated VATS for ILD lung biopsy. ILD is relatively rare, and few reports are available on the epidemiology of ILDs. Karakatsani et al. reported that the estimated annual incidence of ILDs is 4.63 per 100,000 and that the prevalence is 17.3 per 100,000 in Greece (20). Surgical lung biopsy may be required for the diagnosis of ILD. However, because of the morbidity and mortality associated with VATS for ILD, biopsy is not always safe (21-24). According to one single center study (21), among patients who underwent VATS lung biopsy between 1998 and 2004, the 60-day mortality rate was 3 in 68 (4.4%). All three patients were cases of UIP. Two of these patients underwent postmortem examination, and both had diffuse alveolar damage on a background of UIP, consistent with acute exacerbation of UIP. The relationship between positive pressure ventilation and acute exacerbation of ILD has not been clearly elucidated, but postoperative ALI is related to ventilator-induced lung injury (25). In addition, life-threatening pneumothorax was caused by bulla rupture during intubated thoracoscopic lung surgery with a large bulla on the contralateral side (26). Therefore, non-intubated VATS without positive pressure ventilation may eliminate one of the potential triggers of acute exacerbation of ILD after lung biopsy. Several studies demonstrated safety and feasibility of non-intubated VATS lung biopsy in ILD subjects (10-12).
In the current study, a 100% diagnostic success was achieved. Guidelines suggest that more than one specimen should be collected when biopsy is performed (1). After careful examination via high-resolution computed tomography (HRCT), the location of the wedge resection was decided considering the extent and distribution of the lesion. When the wedge resection was performed, the lesion was resected to a size sufficient to include some of the healthy tissue. However, strict standardization of the method used for specimen resection among surgeons is limited.
For limitations, as the total number of subjects was low, it was not possible to meaningfully compare the incidence of death or major complications between the two groups. Additional limitation is the comparability of the groups. Although it was not intentional, there were meaningful differences between the study groups in ASA score and pathological diagnosis. These differences as well as the short time lapse of recruitment of the entire cohort may suggest bias from patient allocation. The patient allocation was decided by surgeons and preference expressed by the patient. Notably, however, their collective outcome was not different. This suggests that non-intubated VATS may be an option in patients with poor lung function for whom general anesthesia is contraindicated.
In the current study, we inserted chest tubes in all subjects. However, as chest tubes cause pain and is closely related to the hospitalization period, chest tubeless VATS lung biopsy can be considered (27). Recent studies have also introduced chest tubeless non-intubated VATS. Guilin Peng et al. reported 43 patients with ILD who underwent chest tubeless non-intubated VATS lung biopsy (11). In such cases, it may be possible to perform the biopsy as an outpatient procedure.
Conclusions
In this study, we successfully performed non-intubated VATS lung biopsy with a facial mask, epidural anesthesia, and conscious sedation. We demonstrated that non-intubated VATS lung biopsy is a safe and feasible procedure even in subjects with high ASA score and poor DLCO. This procedure also resulted in excellent diagnostic yield without any morbidity or mortality. Notably, these preliminary results have to be confirmed by a larger study to assess the long-term effects and diagnostic rate of this less-invasive surgical strategy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the ethics board of Samsung Medical Center (approval number: 2017-09-070). The need for individual patient consent was waived.
References
- American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [PubMed]
- Hutchinson JP, Fogarty AW, McKeever TM, et al. In-Hospital Mortality after Surgical Lung Biopsy for Interstitial Lung Disease in the United States. 2000 to 2011. Am J Respir Crit Care Med 2016;193:1161-7. [Crossref] [PubMed]
- Rotolo N, Imperatori A, Dominioni L, et al. Efficacy and safety of surgical lung biopsy for interstitial disease. Experience of 161 consecutive patients from a single institution in Italy. Sarcoidosis Vasc Diffuse Lung Dis 2015;32:251-8. [PubMed]
- Nguyen W, Meyer KC. Surgical lung biopsy for the diagnosis of interstitial lung disease: a review of the literature and recommendations for optimizing safety and efficacy. Sarcoidosis Vasc Diffuse Lung Dis 2013;30:3-16. [PubMed]
- Papiris SA, Manali ED, Kolilekas L, et al. Clinical review: idiopathic pulmonary fibrosis acute exacerbations--unravelling Ariadne's thread. Crit Care 2010;14:246. [Crossref] [PubMed]
- International consensus conferences in intensive care medicine. Ventilator-associated lung injury in ARDS. American Thoracic Society, European Society of Intensive Care Medicine, Societé de Réanimation Langue Française. Intensive Care Med 1999;25:1444-52. [PubMed]
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338:347-54. [Crossref] [PubMed]
- Dreyfuss D, Saumon G. Role of tidal volume, FRC, and end-inspiratory volume in the development of pulmonary edema following mechanical ventilation. Am Rev Respir Dis 1993;148:1194-203. [Crossref] [PubMed]
- Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med 2004;32:1817-24. [Crossref] [PubMed]
- Pompeo E, Rogliani P, Cristino B, et al. Awake thoracoscopic biopsy of interstitial lung disease. Ann Thorac Surg 2013;95:445-52. [Crossref] [PubMed]
- Peng G, Liu M, Lou Q, et al. Spontaneous ventilation anesthesia combined with uniportal and tubeless thoracoscopic lung biopsy in selected patients with interstitial lung diseases. J Thorac Dis 2017;9:4494-501. [Crossref] [PubMed]
- Katlic MR. Five Hundred Seventy-Six Cases of Video-Assisted Thoracic Surgery Using Local Anesthesia and Sedation: Lessons Learned. J Am Coll Surg 2018;226:58-63. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Dong Q, Liang L, Li Y, et al. Anesthesia with nontracheal intubation in thoracic surgery. J Thorac Dis 2012;4:126-30. [PubMed]
- Shao W, Wang W, Yin W, et al. Nonintubated thoracoscopic lobectomy plus lymph node dissection following segmentectomy for central type pulmonary masses. Chin J Cancer Res 2013;25:124-7. [PubMed]
- Okuda K, Nakanishi R. The non-intubated anesthesia for airway surgery. J Thorac Dis 2016;8:3414-9. [Crossref] [PubMed]
- Kubota T, Miyata A. Postoperative respiratory failure caused by acute exacerbation of idiopathic interstitial pneumonia. J Anesth 2011;25:422-5. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Sellitri F, et al. Surgical stress hormones response is reduced after awake videothoracoscopy. Interact Cardiovasc Thorac Surg 2010;10:666-71. [Crossref] [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [Crossref] [PubMed]
- Karakatsani A, Papakosta D, Rapti A, et al. Epidemiology of interstitial lung diseases in Greece. Respir Med 2009;103:1122-9. [Crossref] [PubMed]
- Kreider ME, Hansen-Flaschen J, Ahmad NN, et al. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg 2007;83:1140-4. [Crossref] [PubMed]
- Sigurdsson MI, Isaksson HJ, Gudmundsson G, et al. Diagnostic surgical lung biopsies for suspected interstitial lung diseases: a retrospective study. Ann Thorac Surg 2009;88:227-32. [Crossref] [PubMed]
- Han Q, Luo Q, Xie JX, et al. Diagnostic yield and postoperative mortality associated with surgical lung biopsy for evaluation of interstitial lung diseases: A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2015;149:1394-401. [Crossref] [PubMed]
- Durheim MT, Kim S, Gulack BC, et al. Mortality and Respiratory Failure After Thoracoscopic Lung Biopsy for Interstitial Lung Disease. Ann Thorac Surg 2017;104:465-70. [Crossref] [PubMed]
- Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med 2006;32:24-33. [Crossref] [PubMed]
- Morcos K, Singh S, Reeve W, et al. Tension Pneumothorax in Contralateral Lung during Left Video Assisted Thoracoscopic Surgery (VATS) Upper Lobectomy. Clin Surg 2016;1:1274.
- Cui F, Liu J, Li S, et al. Tubeless video-assisted thoracoscopic surgery (VATS) under non-intubated, intravenous anesthesia with spontaneous ventilation and no placement of chest tube postoperatively. J Thorac Dis 2016;8:2226-32. [Crossref] [PubMed]


