Treatment of post-thoracic endovascular aortic repair aorto-esophageal fistula—only radical surgery can be effective: techniques and sequence of treatment
Introduction
Aorto-esophageal fistula (AEF) formation occurs with an incidence of approximately 1.5–2% after endovascular repair of the thoracic endovascular aortic repair (TEVAR) (1,2). It has been associated with the presence of mediastinal hematoma at the time of TEVAR, for example on the grounds of a ruptured thoracic aneurysm (1). The underlying pathomechanism may be seen in the increased mediastinal pressure and the inflammation due to hematoma and its resorption. The radial force of the stent-graft against the aortic wall, as well as the change in the geometry of the aorta after TEVAR are also believed to be underlying mechanisms of AEF formation. AEF has been reported to occur from 1 week to more than 4 years after TEVAR (1-7).
Left untreated AEFs are always lethal (1-3). Treatment options include from an esophageal stenting-only approach, to isolated esophagectomy, to esophagectomy followed by radical aortic replacement. The aim of this study is to provide an insight into the current literature on therapeutic options of post-TEVAR AEF and report on the surgical protocol at the authors’ institution over the radical interdisciplinary surgical therapy.
Literature review
Literature on the rare condition of post-TEVAR AEF is scarce. A recent analysis of a multicenter European registry (17 centers) with a total caseload of 2,387 TEVAR procedures identified 36 patients with a post-TEVAR AEF (1). These patients were divided in four groups depending on the treatment approach they received. In the group of patients treated conservatively the 1-year mortality reached 100%. This was in accordance with the findings of other studies (2,3). A second group of patients received esophageal stenting only and had a 17% survival rate after 1 year. In a third group, patients underwent esophagectomy without aortic replacement and the survival rate was 43% at 1 year. Finally, the fourth group underwent both esophagectomy and aortic replacement with stent-graft explantation, and a slight higher 1-year survival rate of 46% could be achieved in this group. Yet, the 1-year survival rates in other published series are shown to reach over 50%, when the radical surgical regimen is applied (4,5). “Redo”-TEVAR as a treatment option has been advocated by some authors, but the long-term results are disappointing in cases of AEF, since control of the infection cannot be sufficiently reached, with the primarily infected stent-graft remaining in place (6). Table 1 summarises the results of the largest series on post-TEVAR AEF therapy.
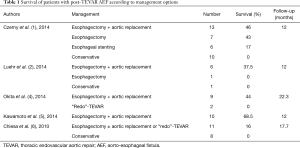
Full table
While the above mentioned analysis of the European registry is the largest comparative study between the different therapeutic approaches of post-TEVAR AEF and nicely demonstrates the survival advantage in the group of patients receiving the most radical approach (esophagectomy and aortic replacement), it has two significant setbacks. As the authors point out, the underlying database failed to demonstrate, if the entire infected aortic wall was removed during stent-graft explantation, and second the material used for aortic replacement was not reported (1).
Currently there are three options regarding the material for replacement of the thoracic aorta: first, polyester grafts (silber-impregnated or antibiotic-soaked); second, homografts; third, self-made tube grafts from bovine pericardial patches (xenografts). There are no comparative data between the different materials for aortic reconstruction. Nevertheless, it is safe to say that polyester grafts are at higher risk for reinfection than homografts or xenografts in this setting (2). On the other hand, homografts are not off-the self available, might happen to be too short or too small in diameter and are prone to aneurysmatic dilatation during follow-up.
European vascular center Aachen-Maastricht approach—techniques and sequence of treatment
The authors’ favorable strategy for treating post-TEVAR AEF includes esophagectomy followed by stent-graft removal and aortic replacement, followed by second-stage esophageal reconstruction (8). We would like to highlight some technical aspects in the following section.
In hemodynamically unstable patients we advocate “redo”-TEVAR as a “bridging” procedure in order to stabilise the patient prior to definitive treatment. The last is in our opinion radical surgery and is ideally performed in a multi-stage fashion.
First, the involved segment of the esophagus has to be widely resected, usually through a right-sided thoracotomy (9).
Second, the infected aortic segment has to be replaced after complete explantation of all prosthetic material (endograft). Most commonly the descending thoracic aorta is involved and thus the aortic replacement can be performed by a left-sided anterolateral thoracotomy through the 5th or 6th intercostal space. For this, the patient is positioned on a vacuum mattress at a right angle (80 to 90 degrees) to the edge of the table and the left hip at 60 degrees (Crawford position). Extracorporeal circulation for distal aortic perfusion is commonly established by femoral artery and femoral vein cannulation. Our operative protocol further includes intubation with a double lumen endotracheal tube, cerebrospinal fluid drainage, intraoperative monitoring of motor evoked potentials (MEPs), and mild hypothermia of 32 to 33 °C (10).
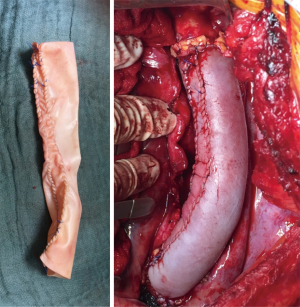
We prefer to reconstruct the resected thoracic aorta by a tube graft self-made from a bovine pericardial patch. Such reconstructions have been also performed by other groups with very good results (2,11). Bovine pericardial patches are off-the self available as 10 cm wide to 16 cm long patches (XenoSure Biologic Patch, LeMaitre Vascular, Sulzbach, Germany). The diameter of the “neo-tube” can be determined by the surgeon and if a longer segment of the aorta requires reconstruction, two or even more such tubes can be sutured together (Figure 1).
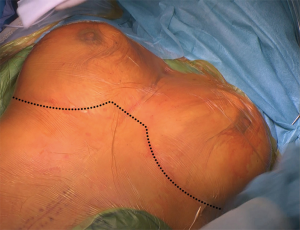
In patients, in whom not only the descending aorta, but also the ascending aorta and the aortic arch require reconstruction, one might consider a bilateral 5th intercostal anterior thoracotomy to access the chest (Figure 2). This so-called “clam-shell” approach enables a complete exposure of the aortic root, aortic arch and descending aorta (Figure 3) (12). We perform replacement of the aortic arch and/or the ascending aorta in moderate (25 °C) hypothermic circulatory arrest, with antegrade cerebral perfusion, and with cerebral and spinal neuromonitoring (MEPs, transcranial Doppler sonography and electroencephalography).
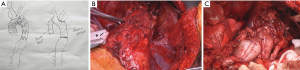
Once the infected aorta has been replaced and the patient has recovered, esophageal reconstruction can be achieved by gastric pull-up through a retrosternal route (13). In the authors’ opinion, following the herein surgical protocol antibiotic therapy can be discontinued before hospital discharge in patients after complete removal of the infected stent-grafts and without use of alloplastic material for the aortic reconstruction.
In the last 10 years, we treated four patients with post-TEVAR AEF, as reported above, and observed an in-hospital mortality rate of 25% (1/4), with the 1-year survival rate being 50% (2/4).
Conclusions
AEFs are rare but lethal complications after TEVAR. “Redo”-stent-grafting can serve as a “bridging” procedure in hemodynamically unstable patients. In the authors’ opinion, radical surgical therapy including esophagectomy, stent-graft removal and aortic replacement followed by esophageal reconstruction represents the treatment of choice in these patients in terms of long-term effectiveness and durability.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Czerny M, Eggebrecht H, Sodeck G, et al. New insights regarding the incidence, presentation and treatment options of aorto-oesophageal fistulation after thoracic endovascular aortic repair: the European Registry of Endovascular Aortic Repair Complications. Eur J Cardiothorac Surg 2014;45:452-7. [Crossref] [PubMed]
- Luehr M, Etz CD, Nozdrzykowski M, et al. Emergency open surgery for aorto-oesophageal and aorto-bronchial fistulae after thoracic endovascular aortic repair: a single-centre experience. Eur J Cardiothorac Surg 2015;47:374-82; discussion 382-3. [Crossref] [PubMed]
- Canaud L, Ozdemir BA, Bee WW, et al. Thoracic endovascular aortic repair in management of aortoesophageal fistulas. J Vasc Surg 2014;59:248-54. [Crossref] [PubMed]
- Okita Y, Yamanaka K, Okada K, et al. Strategies for the treatment of aorto-oesophageal fistula. Eur J Cardiothorac Surg 2014;46:894-900. [Crossref] [PubMed]
- Kawamoto S, Sato M, Motoyoshi N, et al. Outcomes of a staged surgical treatment strategy for aortoesophageal fistula. Gen Thorac Cardiovasc Surg 2015;63:147-52. [Crossref] [PubMed]
- Chiesa R, Melissano G, Marone EM, et al. Aorto-oesophageal and aortobronchial fistulae following thoracic endovascular aortic repair: a national survey. Eur J Vasc Endovasc Surg 2010;39:273-9. [Crossref] [PubMed]
- Cheng L, Zhu J, Liu X, et al. A Successful Three-Stage Surgical Treatment for Aortoesophageal Fistula After Thoracic Endovascular Aortic Repair and Esophageal Stent Repair. Ann Thorac Surg 2016;102:e503-5. [Crossref] [PubMed]
- Gombert A, Grommes J, Schick G, et al. Sarcoidosis-Associated Aortoesophageal Fistula-Multistage Interdisciplinary Surgical Therapy for a Rare and Life-Threatening Condition. Ann Vasc Surg 2017;39:287.e15-287.e20. [Crossref] [PubMed]
- Kim SH, Lee KS, Shim YM, et al. Esophageal resection: indications, techniques, and radiologic assessment. Radiographics 2001;21:1119-37; discussion 1138-40. [Crossref] [PubMed]
- Jacobs MJ, Schurink GW. Open repair in chronic type B dissection with connective tissue disorders. Ann Cardiothorac Surg 2014;3:325-8. [PubMed]
- Zientara A, Schwegler I, Dzemali O, et al. Xenopericardial self-made tube grafts in infectious vascular reconstructions: Preliminary results of an easy and ready to use surgical approach. Vascular 2016;24:621-7. [Crossref] [PubMed]
- Hino Y, Okada K, Oka T, et al. Extended replacement of the thoracic aorta. Eur J Cardiothorac Surg 2013;43:176-81; discussion 181. [Crossref] [PubMed]
- Yildirim S, Köksal H, Celayir F, et al. Colonic interposition vs. gastric pull-up after total esophagectomy. J Gastrointest Surg 2004;8:675-8. [Crossref] [PubMed]


