Esophagectomy after endoscopic submucosal dissection for esophageal carcinoma
Introduction
Endoscopic submucosal dissection (ESD) is used to treat patients who have esophageal carcinoma and to stage the tumor by determining tumor invasion depth. In addition, ESD may help better understand the esophageal microvasculature in adenocarcinoma and squamous cell carcinoma (1-3). Furthermore, ESD enables targeted endoscopic removal of superficial tissue of the gastrointestinal tract, with acceptable complication risks and favorable long-term outcomes (4,5). The indications for ESD in treating early stage esophageal carcinoma include lesion depth not exceeding submucosa (tumor confined to the mucosa and superficial submucosa), and absence of lymph node involvement (4,6).
The frequency of complete resection (R0; tumor negative margin) of esophageal cancer with ESD could achieve 91.7% to 97% (4). Commonly seen complications after ESD include esophageal perforation, pneumonia, bleeding, and esophageal stricture (5). Delayed bleeding after ESD is rare, but immediate bleeding after ESD may occur in 1.6% to 10% patients (4,5). Esophageal stricture after ESD may occur in 13.9% patients (5,7). The 5-year overall, recurrence-free, and cause-specific survival rates after ESD treatment for esophageal cancer are 94.2%, 92.3%, and 96.1% (8). In a small review of nine patients, esophagectomy after ESD provided high rates of cure with acceptable safety, and was considered useful (9). However, the indications and outcomes of subsequent esophagectomy after ESD are unknown.
A portion of patients with superficial esophageal cancer can be cured by less invasive treatment of endoscopic dissection, and ESD can provide high cure rate with acceptable safety. As we known complete removal of cancer tissue together with lymph nodes was the advantage of esophagectomy, however, invasiveness, organ loss, perioperative complications, and worse postoperative quality of life were serious disadvantages. The purpose of the present study was to investigate the indications for additional esophagectomy after ESD. We aimed to find the patients who indeed required additional esophagectomy after ESD and help the other patients avoid unnecessary and excessive surgery.
Methods
Patients
The clinicopathologic data were reviewed for consecutive patients who had esophageal cancer and were treated with ESD and subsequent esophagectomy between October 2011 and December 2016 in our thoracic surgery department. Esophageal cancer was confirmed by endoscopic biopsy before ESD, and any noncancer patient was excluded. Each patient had a computed tomography (CT) scan of the chest and epigastrium with contrast and endoscopic ultrasonography (EUS) before ESD. Contraindications for ESD included tumor involvement deep to the esophageal submucosa or lymph node metastasis indicated by CT scan and EUS. All patients in this study signed informed consents. This study was approved by Ethics Committee of West China Hospital, Sichuan University (No. 2011-63).
ESD
The ESD procedure was performed under general anesthesia with endotracheal intubation, and included4steps: (I) the gross lesion was marked circumferentially with Lugol solution, and the intended dissection area was marked with an electrosurgical knife; (II) fluid (1:10,000 epinephrine saline + sodium hyaluronate + indicarmine + glycerol fructose) was injected submucosally to elevate the lesion along the marked border; (III) the border of the mucosal incision was marked; and (IV) en bloc submucosal dissection was performed. The proper muscular layer was evaluated carefully to verify absence of injury, and a nasogastric tube was placed. When there were no abnormal symptoms or complications, oral fluid intake was started, and the nasogastric tube was removed on the day after surgery.
The esophageal specimen resected by ESD was sectioned at 2-mm intervals. Complete resection was defined histologically when the tumor had been resected en bloc with cancer-negative margins.
Esophagectomy: surgical indications and procedure
The indications for esophagectomy after ESD were analyzed to determine the necessity of surgery. The main indications for esophagectomy after ESD were varied because clear guidelines were lacking. The indications in our series included non-pathologic (ESD failure, refractory stricture, urgent treatment required for esophageal rupture) and pathologic factors (T1b stage, vascular invasion, tumor present at the ESD margin, extensive or multiple lesions, recurrence) and the patient’s treatment intention of surgery. We defined esophagectomy necessity to mean that esophagectomy was inevitable after ESD, or residual cancer or lymph node metastasis after surgery was confirmed. And the esophagectomy necessity outcomes were retrospectively analyzed to judge whether the surgery option was correct. We aimed to select the patients who required further treatment after ESD and help the other patients avoid unnecessary surgery.
Esophageal edema that was observed at esophagectomy after ESD was classified in four stages: none, mild (edema limited to ESD wound), moderate (edema limited to esophageal wall), and severe (periesophageal and pleural edema causing difficult resection). The edema usually located at ESD site or extending to esophageal wall and periesophageal pleural. The esophageal edema could increase the difficulty in distinguishing and separating the esophagus from adjacent tissues. Open or minimally invasive esophagectomy and 2-field lymphadenectomy were performed under general anesthesia with a double-lumen tube for endotracheal intubation. Tubular stomach was chosen as substitution for reconstruction and esophagogastrostomy was performed at intrathoracic or cervical location. Oral fluid intake was started on postoperative day 6 or 7 when an esophagogram verified esophagogastric integrity and there was no complication. A follow-up examination was generally scheduled every 3 months for the first two year after ESD or esophagectomy, every twice yearly for the 3rd to 5th year, and annually thereafter. The regular follow-up assessment included physical examination, contrast CT scan, esophagography, and endoscopy.
Data analysis
Data analysis was performed with statistical software (SPSS 16.0 for Windows, SPSS Inc., Chicago, IL, USA). Categorical data were analyzed with chi-square or Fisher exact test. Ordinal data were compared with Kruskal-Wallis test. Independent t-test was used to analyze continuous data such as age and tumor length. Unconditional logistic regression was used to estimate the factors associated with esophagectomy necessity. Statistical significance was defined by P<0.05.
Results
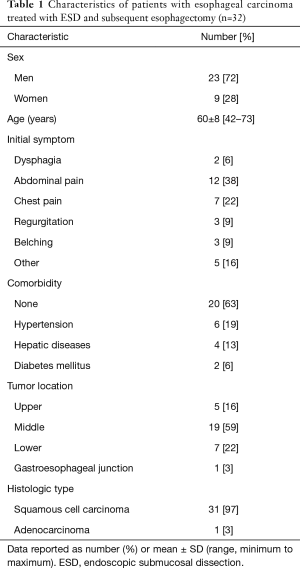
Total 214 patients with esophageal and esophagogastric cancer have undergone ESD treatment in our center, of which 32 patients ultimately required esophagectomy after ESD (Table 1). The initial symptom was dysphagia in only 2 patients (6%); other patients presented with abdominal pain (12 patients), chest pain (7 patients), regurgitation (3 patients), belching (3 patients) and other symptoms (5 patients). Esophageal cancer was confirmed by endoscopic biopsy before ESD. Most tumors were squamous cell carcinomas in the middle esophagus (Table 1), and mean endoscopic tumor length was 6±3 cm (range, 1–13 cm).
Full table
ESD
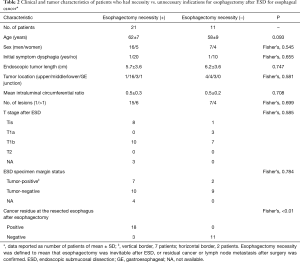
The ESD procedure could not be completed in 3 patients because of severe hypoxemia (1 patient), tachyarrhythmia (1 patient), and proper muscular involvement (1 patient); in these patients, ESD was cancelled, and no dissected cancer tissue was obtained for pathologic examination. There was no severe morbidity or death during ESD. In 1 patient (3%) who had lower esophageal cancer, postoperative hemorrhage after ESD occurred at the dissection site that healed with non-operative treatment and no blood transfusion. There were 2 patients who had revision ESD at 4 and 6 months after the first ESD because of tumor present at the ESD margin. After ESD, most patients had tumor-negative margins and tumor stage T1b (Table 2). Among other 182 patients that underwent ESD alone, the pT stage according to ESD specimen included Tis (86 patients), T1a (71 patients) and T1b (25 patients).
Full table
Esophagectomy
The main reasons for performing esophagectomy after ESD included T1b stage (12 patients: T1b stage alone, 6 patients; T1b stage with positive margin, 3 patients (cancer, 2 patients; intraepithelial neoplasia, 1 patient); T1b stage with refractory stricture, 2 patients; T1b stage with vascular invasion, 1 patient); positive margin alone (7 patients); recurrence (4 patients); ESD failure (3 patients); extensive or multiple lesions (3 cases); basal layer invasion (2 patients); and urgent surgery for esophageal rupture (1 patient). Esophagectomies were performed with minimally invasive esophagectomy (19 patients, including 1 patient who had open thoracotomy because of severe esophageal and mediastinal edema), left thoracotomy (12 patients), and open McKeown esophagectomy (1 patient). Esophagogastrostomy was by circular stapler at the intrathoracic (12 patients) and hand-sewn at the cervical level (20 patients). Mean operative blood loss was 184±65 mL (range, 90–310 mL), and no blood transfusion was needed. The most important finding at esophagectomy after ESD was esophageal edema, caused by the previous ESD wound that increased the difficulty of performing esophagectomy.
Median hospital stay after esophagectomy was 10 days (range, 8–53 days). Postoperative complications in 11 patients (34%) included 4 pulmonary (pleural effusion, pneumonia, tracheobronchial injury), 3 gastrointestinal (anastomotic leak, conduit necrosis, liver dysfunction), 3 chylothorax, 1 deep venous thrombosis, according to the Esophagectomy Complications Consensus Group (ECCG) system (10). No patient died of complications after esophagectomy with the in-hospital and 30-day mortality of 0%.
Esophagectomy resulted in complete resection (R0) in all patients. The pathologic TNM stages after esophagectomy were TisN0M0 (6 patients), T1aN0M0 (6 patients), T1bN0M0 (18 patients), T1bN1M0 (1 patient), and T2N3M0 (1 patient). The pT stage was similar after esophagectomy vs. after ESD. There were mean 16±7 lymph nodes excised (range, 6–31 lymph nodes); lymph node metastasis was observed in 1 patient (T1b stage) who had upper esophageal squamous cell carcinoma (station 2 lymph node positive) and 1 patient (T2 stage) who had gastroesophageal junction adenocarcinoma (station 16 and 17 lymph nodes positive). Cancer residue was verified pathologically at the resected esophagus in 18 patients (56%).
Indications for esophagectomy
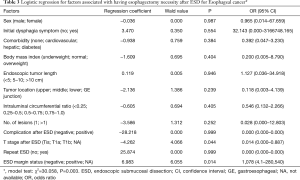
Failure of ESD, cancer recurrence, emergency treatment for esophageal rupture, and stricture that was refractory to dilation were considered absolute indications for esophagectomy (10 patients). Esophagectomy also was considered necessary for post-esophagectomy residual cancer in either the resection margin or regional lymph nodes (11 patients). There were 21 patients (65.6%) for whom esophagectomy was considered necessary (Table 2). There was no difference in clinicopathologic factors between patients for whom esophagectomy was necessary vs. unnecessary after ESD (Table 2). Therefore, further analysis was performed for the subgroup excluding the 10 patients with absolute esophagectomy indications clearly judged before surgery. In the remaining 22 patients, 11 patients had esophagectomy that was considered necessary (stage Tis, 3 patients; T1b, 8 patients) and other 11 patients underwent esophagectomy that was considered unnecessary (stage Tis, 1 patient; T1a, 3 patients; T1b, 7 patients); there was no difference in T stage after ESD between these 2 patient groups (not significant). However, there was a significant difference in ESD specimen margin status between the 2 groups (positive/negative margin: 8/3 vs. 2/9 patients; P=0.03). Logistic regression showed that T stage and ESD specimen margin status were associated with esophagectomy necessity after ESD (Table 3). The differences between T stages were not significant for ESD failure, complications after ESD, lymph node metastasis, positive tumor margin after ESD, and esophagectomy necessity (Table 4).
Full table

Full table
Interval between ESD and esophagectomy
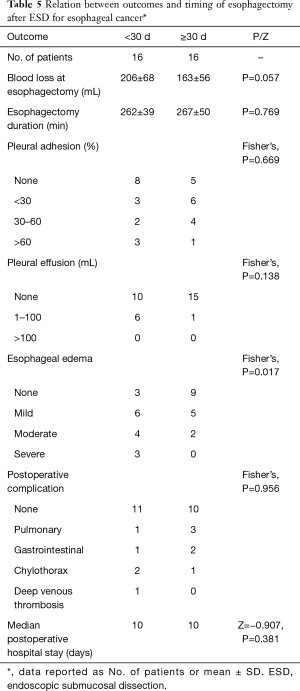
Esophageal edema that was observed at esophagectomy after ESD increased operative difficulty during esophageal resection, lymphadenectomy and anastomosis according to our practice, but the edema seemed less severe at longer intervals between ESD and esophagectomy. Meanwhile, the blood loss may be more if edema is more severe (206±68 vs. 163±56 mL, P=0.057). Esophagectomy performed ≥30 days after ESD was associated with less severe esophageal edema than earlier esophagectomy (Table 5).
Full table
Postoperative outcome
Most recent follow-up was at median 16.8 months (range, 11.2–54.5 months). There were 3 patients who were lost to follow-up (9.4%), and 1 other patient (stage, pT1bN1M0; upper squamous cell carcinoma) died of metastatic disease at after esophagectomy. Among other 182 patients that underwent ESD alone, 14 patients were lost to follow-up (7.7%), 2 patients (T1b) died of metastatic disease, and 1 patient died of hepatic cirrhosis. All other patients were alive with excellent postoperative disease-free survival.
Discussion
Esophageal ESD is safe and feasible and has acceptable morbidities and favorable long-term outcomes for treatment of early stage esophageal cancer. However, the goals of ESD include removal of the lesion en bloc and to save the patient from esophageal cancer-related death (11). In our study, in-hospital morbidity of ESD occurred in 3 patients (9.4%) including one with bleeding, one with hypoxemia and one with tachyarrhythmia, and there were no deaths. T stage evaluated from the ESD-resected specimen was similar to the pathologic T status after esophagectomy. The ESD resection may accurately determine the depth of tumor invasion, and maybe used as a staging procedure in early stage esophageal cancer (1).
In our study, we defined esophagectomy necessity to mean that esophagectomy was inevitable after ESD (failure of ESD, cancer recurrence, esophageal rupture, and refractory stricture), or residual cancer or lymph node metastasis after surgery was confirmed. The problems above could be solved by esophagectomy + lymphadenectomy rather than endoscopic treatment, chemotherapy or radiation. And the esophagectomy necessity outcomes were retrospectively analyzed to judge whether the surgery option was correct. Overall 21 patients (65.6%) had esophagectomy necessity. For the other 11 patients, esophagectomy after ESD was not mandatory. Failure of ESD, cancer recurrence, emergency surgery for esophageal rupture, and stricture refractory to dilation were considered absolute indications to esophagectomy; for these patients, esophagectomy was inevitable and the most effective treatment option. For the patients who had recurrent cancer after ESD, the normal anatomic structure of the mucosa and submucosa were absent at endoscopic examination, and tumor invasion depth could not be clearly observed and determined with repeat ESD. There was one urgent esophagectomy for sudden esophageal rupture that occurred during endoscopic dilation to treat esophageal stricture after ESD; suture repair of the esophagus could not be performed because of severe edema, inflammation, and a transmural defect of mucosa. Esophageal stricture is a commonly observed complication after ESD. Refractory stricture after ESD may be associated with muscle layer damage over a long longitudinal mucosal defect (>5 cm) with circumferential extent >75% (12,13). Corticosteroid treatment by injection or oral intake, together with endoscopic dilation, may help prevent stricture after ESD (14,15). However, refractory stricture could increase the risk of developing esophageal perforation during or after endoscopic balloon dilation (13). In the present study, two patients had esophagectomy because of stricture after ESD that was refractory to dilation.
A multicenter study showed that significant differences in 5-year disease-free survival rates were observed between patients who had curative vs. noncurative resections after ESD (84.8% vs. 72.7%; P<0.01) (5). The relapse-free survival rate was greater in patients who received (88%) than did not receive additional treatment (64%) (P=0.04) during 3-year follow-up after ESD for esophageal cancer (16) Therefore, esophagectomy after ESD is necessary for the patients who were not curative by ESD, to provide radical treatment for early stage esophageal cancer. Although the combination of ESD and chemoradiation is an effective approach that could offer good long-term survival for esophageal squamous cell carcinoma at M3 or stage T1b determined by histologic assessment, chemoradiation is limited to patients who have poor health status and cannot tolerate surgery (17).
In the present study, esophagectomy necessity after ESD was associated with T stage and residual margin status (Table 3), and T stage alone was not an indication for further resection of the esophagus (Table 4); however, T1b stage may be associated with other factors (positive margin, stricture) that required esophagectomy. The maximum diameter of the resected specimens and the depth of tumor invasion are risk factors for positive resection margins after ESD, suggesting that larger lesions and a greater depth of invasion increases the frequency of residual tumor after ESD (18). Therefore, stage T1b is associated with higher positive margin rate. In a previous study, additional esophagectomy after ESD for patients who had stagepT1b cancer was considered valid treatment because it provided high curative rates with acceptable safety; however, the conclusion may be guarded because the study was limited to nine patients (9). Therefore, we suggest that esophagectomy may not be recommended for stage T1b alone or an earlier stage with negative margin in the ESD specimen.
Lymph node metastasis is another issue concerning surgical necessity. The prevalence of lymph node metastases in esophageal adenocarcinoma, squamous cell carcinoma, and junctional adenocarcinoma was 6.4%, 6.9%, and 9.5% for pT1a tumors and 19.6%, 20%, and 22.9% for pT1b tumors (19). The frequency of lymph node metastasis was 0% in m1 and m2, 9% in m3, 16% in sm1, 35% in sm2, and 62% in sm3 patients (20). The higher frequency of lymph node involvement in T1b cancers may preclude the use of endoscopic treatment except for patients unfit for surgery (21). The lymph node metastasis frequency in our cohort was 0% in stage Tis and T1a, and 5.3% (1 in 19 patients) in stage T1b (Table 4). Mean number of excised lymph nodes (16±7 lymph nodes) was greater than that recommended by NCCN guidelines and may enable accurate assessment of N stage (22). Lymph node status should be evaluated carefully by CT, EUS, or positron emission tomography before ESD to exclude undiagnosed lymph node metastasis. However, in this study, the lymph node metastasis frequency was 0% (Tis and T1a), and 5.3% (T1b) in the cohort of 32 patients, but the lymph nodal pathological status of other 182 patients undergoing ESD alone was not available. This is the shortcoming of this study and it’s greatly necessary to evaluate the lymph node carefully for the ESD alone patients.
According to our practice, minimally invasive esophagectomy with cervical esophagogastrostomy was the optimum surgical procedure. The upper stump of the esophagus should have a safe distance away from the ESD site to guarantee esophageal mucosal quality and anastomotic reliability. We observed esophageal edema caused by ESD that could increase surgical difficulty and operative blood loss; therefore, esophagectomy should be delayed at least 30 days after ESD to enable resolution of esophageal edema. However, other objective parameters (operation duration, postoperative complication, hospital stay) were not influenced by the edema status. We will further research the esophageal edema after ESD and find more relevant objective parameters. Post-esophagectomy morbidity was similar between the present and a previous study (23). In another report, no recurrence was observed in any patient who had additional surgical resection after histologic noncurative ESD for treatment of superficial adenocarcinoma of the esophagogastric junction (24). In the present study, the survival after esophagectomy following ESD was excellent, but further investigation is needed to verify the present findings.
The short follow-up was the disadvantage in this study, because ESD has been performed treating esophageal cancer in our hospital in recent years. Further follow-up and survival outcome collecting is still carried out. Meanwhile, the volume of this study was limited and large-scale study on ESD and following esophagectomy was needed.
Conclusions
Indications for esophagectomy after ESD include ESD failure, cancer recurrence, esophageal rupture, esophageal stricture refractory to endoscopic dilation, and residual tumor at the ESD specimen margin. Stage T1b alone is not an indication for esophagectomy. According to our study, we recommend that esophagectomy should be delayed ≥30 dafter ESD unless urgent esophagectomy is indicated.
Acknowledgements
The first author thanked Pei-Yan Wang for her kind support in the manuscript writing.
Funding: This research was supported by Science and Technology Bureau of Chengdu (Grant # 2015-HM01-00395-SF) (the funding source had no role in study design, data collection and analysis, manuscript drafting and decision to submit for publication).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of West China Hospital, Sichuan University (No. 2011-63) and written informed consent was obtained from all patients.
References
- Maish MS, DeMeester SR. Endoscopic mucosal resection as a staging technique to determine the depth of invasion of esophageal adenocarcinoma. Ann Thorac Surg 2004;78:1777-82. [Crossref] [PubMed]
- Maselli R, Inoue H, Ikeda H, et al. Microvasculature of the esophagus and gastroesophageal junction: lesson learned from submucosal endoscopy. World J Gastrointest Endosc 2016;8:690-6. [Crossref] [PubMed]
- Probst A, Aust D, Markl B, et al. Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy 2015;47:113-21. [PubMed]
- Evans JA, Early DS, Chandraskhara V, et al. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328-34. [Crossref] [PubMed]
- Tsujii Y, Nishida T, Nishiyama O, et al. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: a multicenter retrospective cohort study. Endoscopy 2015;47:775-83. [Crossref] [PubMed]
- Shah PM, Gerdes H. Endoscopic options for early stage esophageal cancer. J Gastrointest Oncol 2015;6:20-30. [PubMed]
- Park JS, Youn YH, Park JJ, et al. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal squamous neoplasms. Clin Endosc 2016;49:168-75. [Crossref] [PubMed]
- Yamada M, Oda I, Nonaka S, et al. Long-term outcome of endoscopic resection of superficial adenocarcinoma of the esophagogastric junction. Endoscopy 2013;45:992-6. [Crossref] [PubMed]
- Nako Y, Shiozaki A, Fujiwara H, et al. Esophagectomy after endoscopic submucosal dissection (ESD) . Gan To Kagaku Ryoho 2014;41:1997-9. [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg. 2015;262:286-94. [Crossref] [PubMed]
- Fujishiro M, Kodashima S, Goto O, et al. Endoscopic submucosal dissection for esophageal squamous cell neoplasms. Dig Endosc 2009;21:109-15. [Crossref] [PubMed]
- Miwata T, Oka S, Tanaka S, et al. Risk factors for esophageal stenosis after entire circumferential endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Surg Endosc 2016;30:4049-56. [Crossref] [PubMed]
- Hanaoka N, Ishihara R, Uedo N, et al. Refractory strictures despite steroid injection after esophageal endoscopic resection. Endosc Int Open 2016;4:E354-9. [Crossref] [PubMed]
- Yu JP, Liu YJ, Tao YL, et al. Prevention of esophageal stricture after endoscopic submucosal dissection: a systematic review. World J Surg 2015;39:2955-64. [Crossref] [PubMed]
- Ono S, Fujishiro M, Niimi K, et al. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy 2009;41:661-5. [Crossref] [PubMed]
- Ikeda A, Hoshi N, Yoshizaki T, et al. Endoscopic submucosal dissection (ESD) with additional therapy for superficial esophageal cancer with submucosal invasion. Intern Med 2015;54:2803-13. [Crossref] [PubMed]
- Kawaguchi G, Sasamoto R, Abe E, et al. The effectiveness of endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer. Radiat Oncol 2015;10:31. [Crossref] [PubMed]
- Wen J, Linghu E, Yang Y, et al. Relevant risk factors and prognostic impact of positive resection margins after endoscopic submucosal dissection of superficial esophageal squamous cell neoplasia. Surg Endosc 2014;28:1653-9. [Crossref] [PubMed]
- Dubecz A, Kern M, Solymosi N, et al. Predictors of lymph node metastasis in surgically resected T1 esophageal cancer. Ann Thorac Surg 2015;99:1879-85; discussion 1886.
- Akutsu Y, Uesato M, Shuto K, et al. The overall prevalence of metastasis in T1 esophageal squamous cell carcinoma: a retrospective analysis of 295 patients. Ann Surg 2013;257:1032-8. [Crossref] [PubMed]
- Fovos A, Jarral O, Panagiotopoulos N, et al. Does endoscopic treatment for early oesophageal cancers give equivalent oncological outcomes as compared with oesophagectomy? Best evidence topic (BET). Int J Surg 2012;10:415-20. [Crossref] [PubMed]
- Hu Y, Hu C, Zhang H, et al. How does the number of resected lymph nodes influence TNM staging and prognosis for esophageal carcinoma? Ann Surg Oncol 2010;17:784-90. [Crossref] [PubMed]
- Wang WP, Gao Q, Wang KN, et al. A prospective randomized controlled trial of semi-mechanical versus hand-sewn or circular stapled esophagogastrostomy for prevention of anastomotic stricture. World J Surg 2013;37:1043-50. [Crossref] [PubMed]
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010;72:960-6. [Crossref] [PubMed]


