Preoperative evaluation for lung cancer resection
Introduction
During the last decades lung cancer is the leading cause of death worldwide for both sexes (1). Even though cigarette smoking has been proved to be the main causative factor, many other agents [e.g., occupational exposure to asbestos (2) or heavy metals (3), indoor exposure to radon gas radiation (4), particulate air pollution (5)] have been associated with its development.
Even though there are no randomized, controlled studies comparing surgical to non-surgical treatment options for early stage lung cancer, retrospective analyses showed that surgery is the most effective radical treatment approach (6,7). Lung cancer usually causes symptoms when the disease is locally advanced or metastatic. Recently screening programs proved to reduce mortality among heavy-smokers (8) although establishment of such strategies in everyday clinical practice is much more difficult and unknown if it is cost effective compared to other neoplasms (e.g., breast or prostate cancer). Adding severe comorbidities (coronary heart disease, COPD) to the above reasons as cigarette smoking is a common causative factor, we could explain the low surgical resection rates (approximately 20-30%) for lung cancer patients (9-11).
Age alone is not a contraindication and nowadays about one third of patients undergoing surgical resection for lung cancer are >70 years old (12). Elderly should be treated according to the same therapeutic algorithms as younger patents based on holistic evaluation and calculated risk rates. After a detailed staging plan (PET-CT, EBUS/EUS guided TBNA) patients with limited disease should undergo evaluation for their cardiorespiratory reserves so an informed decision could be taken based on objective measurements and risk/benefits ratio. Three clinical guidelines reports of different associations have been published [American College of Chest Physisians (12), British Thoracic Society (13) and European Respiratory Society/European Society of Thoracic Surgery (14)] providing detailed algorithms for preoperative assessment.
Perioperative morbidity and mortality
The extent of lung resection is strongly associated with mortality, with pneumonectomy demonstrating 2-3 times higher mortality compared to lobectomy (15,16). Thirty-days mortality for lobectomy was 2.3% and 7% for pneumonectomy according to data of National Lung Cancer Audit in England from 2004 to 2010 (10,991 patients) (17). A database analysis among 18,800 lung cancer resections showed that significant prognostic factors for mortality were: pneumonectomy, bilobectomy, American Society of Anesthesiologists (ASA) Physical Status Scale rating, Zubrod performance status score, renal dysfunction, induction chemoradiation therapy, steroid use, older age, urgent procedures, male gender, forced expiratory volume in 1 second (FEV1), and body mass index (18).
Controversial results exist about mortality among the elderly (19,20) but all guidelines recommend that careful selection of patients >75-80 years old—based on objective measurements—is mandatory. Thoracic Surgery Scoring System (Thoracoscore) is a well-validated, multidimensional tool (21) that includes nine variables (Table 1) and could predict the risk of death with acceptable accuracy for clinical practice. Such tools could be used as part of a medical interview to quantify the perioperative risk to the patient and enable him to take an informed decision.
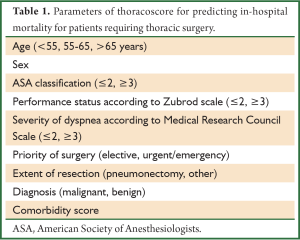
Full table
We should emphasize that surgical experience is an important contributing factor as both surgeon’s expertise (thoracic surgeons compared to general surgeons) as well as hospital-volume of procedures have been associated with perioperative mortality, resectability rates and long-term survival (22-24).
Postoperative morbidity reaches 40% while the main adverse are: atrial arrhythmias, prolonged chest tube drainage and air leak, respiratory complications (sputum retention, pneumonia, empyema), hemorrhage, wound infection, chylothorax and recurrent laryngeal nerve injury (25,26).
Assessment of cardiovascular risk
Guidelines (12-14) agrees that cardiovascular evaluation should be the first step across preoperative assessment for lung cancer resection. Taking into account that the majority of these patients are over 60 years old and current/former smokers, they usually have prior diagnosis of heart disease or represent a potentially high-risk population. Major cardiac-related adverse events are reported in 2-3% of patients postoperatively (27). Thoracic revised cardiac risk index (ThRCRI) is a validated tool (27) that includes four parameters (pneumonectomy, 1.5 points; previous ischemic heart disease, 1.5 points; previous stroke or transient ischemic attack, 1.5 points; creatinine >2 mg/dL, 1 point) and could be used to categorize patients. Those with >1.5 points, recent diagnosis of active heart disease (e.g., unstable angina, severe aortic stenosis, significant arrhythmias) or limited exercise capacity should be sent for cardiologic consultation. Medical history for risk factors, clinical examination, noninvasive evaluation for coronary artery disease—preferably using cardiopulmonary exercise testing—as well as echocardiography for quantification of ventricular and valves function are usually indicated. Myocardial infarction within the last 30 days is considered as a contraindication for the resection (13).
We should continue prior medication (β-blockers, antiplatelet agents, statins) and initiate them if there is an indication independent of the surgery in very high-risk patients. Use of clopidogrel does not increase the possibility of bleeding in the perioperative period while patients with a coronary artery stent should not stop it (28).
Prophylactic use of extended-release β-blockers is not indicated as in a randomized study it increased both mortality and stroke rate (29). Similarly prophylactic coronary artery revascularization was not associated with short or long-term positive results even though current data are referred to patients who undergo major vascular surgery (30,31). Coronary artery revascularization is proposed when there is an absolute indication irrespective of the planned lung cancer resection (14).
Assessment of respiratory function and exercise capacity
It is strongly recommended that all patients who are candidates for surgical treatment of lung cancer should undergo both spirometry (FEV1) and measurement of diffusing capacity for CO (DLco) (12-14). As these two parameters represent function of different lung compartments (FEV1 is mainly associated with airflow limitation while DLco describes function of the alveolar-capillary membrane), it has been proved that they are not strongly correlated (32). Preoperative FEV1 and DLco as well as calculated predicted postoperative (ppo) have been independently associated with morbidity and mortality rates in several studies (33-37). It is interesting to mention that patients with COPD demonstrate only a slight loss or even increase in respiratory function parameters after surgery and this is attributed to removal of emphysematous parenchyma around the tumor (“lobar volume reduction effect”) (38,39). So the decision for surgery for COPD patients should be based mainly on exercise capacity tests rather than static lung function measurements.
Calculation of ppo values should be based on perfusion scan for patients undergoing pneumonectomy: ppo FEV1 = preoperative FEV1 X (1-fraction of the total perfusion for the resected lung). On the other hand the following equation is used for lobectomy or segmentectomy: ppo FEV1 = preoperative FEV1 X (19-removed segments-obstructed segments/19-obstructed segments). The same equations are used for ppo DLco. Even though ppo values are correlated with postoperative morbidity (40), they are usually achieved >1 month after surgery (41) and early postoperative FEV1 is a better predictor of survival (42).
Patients with preoperative FEV1 and DLco >80% predicted or ppo FEV1 and ppo DLco >60% predicted are considered of low risk even to undergo pneumonectomy (12,14). If both ppo FEV1 and ppo DLco are <60% predicted then it is necessary to evaluate patient’s exercise capacity. This could be done either with low-cost tests (shuttle walk test, stair-climbing) or with a cardiopulmonary exercise test (CPET). The latter is a maximal exercise test that assesses both respiratory and cardiac response to stress. Maximal oxygen consumption (VO2 max) as well as signs of ischemic heart disease (ECG abnormalities) or heart failure (low anaerobic threshold) is important for the preoperative evaluation. The main disadvantage of this technique is its high cost and is not available in many thoracic surgery departments.
Shuttle walk test and stair-climbing are strongly correlated with CPET (43,44). A cut-off point of 400 m for the former and 22 m for the latter correspond to VO2 max >15 mL/kg/min which is considered enough for performing lobectomy or segmentectomy. Patients with either ppo FEV1 or ppo DLco 30-60% pred. should be evaluated with low-cost exercise tests and if these are above the critical cut-off points then the planned resection is considered of acceptable risk (45). We should mention that stair-climbing test has not been standardized (speed, number of steps per flight, specific criteria for ending the test).
CPET is indicated for patients with limited lung function reserves according to static measurements (ppo FEV1 or ppo DLco <30% pred.) (12). In centers where the test is available we recommend to use it to the majority of the patients (FEV1 or DLco <80%) as published studies about its role on preoperative evaluation are much more than low-cost exercise tests. Patients with VO2 max >20 mL/kg/min or >75% pred. could be undergone even pneumonectomy (46,47). On the contrary those with VO2 max <10-12 mL/kg/min or <35% pred. represent a high risk group and major anatomic resection is contraindicated (48,49). An intermediate risk group is consisted of patients with VO2 max =10-15 mL/kg/min (50,51). In this case an informed decision should be taken from the patient in collaboration with the surgeon or alternative treatments could be discussed (wedge resection, 3D or stereotactic radiotherapy, radiofrequency ablation).
There are three published guidelines papers of different medical societies. ACCP guidelines have a step-by-step approach—mimicking everyday clinical practice for many thoracic surgery departments—starting with lung function measurements, and then propose low-cost exercise capacity tests and finally CPET. On the other hand ERS/ESTS guidelines recommend CPET for those with either FEV1 or DLco <80% which is not available for many centers while ppo values are used later in the algorithm. BTS guidelines give a detailed approach for staging the disease but there are no proposed cut-off points for FEV1 or DLco.
Additionally there are a number of strategies that could reduce perioperative morbidity and mortality in patients with marginal lung function reserves. Multidisciplinary approach is essential in order to implement such an individualized treatment. Smoking cessation (52), VATS lobectomy instead of lateral thoracotomy (53), lung volume reduction surgery simultaneously with tumor resection in COPD patients with emphysema of the upper lobes (54), programs of pulmonary rehabilitation pre and after surgery (55) is important supportive/alternative care for severely compromised patients with underline chronic pulmonary diseases.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Berman DW, Crump KS. A meta-analysis of asbestos-related cancer risk that addresses fiber size and mineral type. Crit Rev Toxicol 2008;38 Suppl 1:49-73. [PubMed]
- Alberg AJ, Yung RC, Strickland P, et al. Respiratory cancer and exposure to arsenic, chromium, nickel and polycyclic aromatic hydrocarbons. Clin Occup Environ Med 2002;2:779-801.
- Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ 2005;330:223. [PubMed]
- Raaschou-Nielsen O, Andersen ZJ, Beelen R, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol 2013;14:813-22. [PubMed]
- Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg 2009;15:4-9. [PubMed]
- Detterbeck FC, Gibson CJ. Turning gray: the natural history of lung cancer over time. J Thorac Oncol 2008;3:781-92. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- Damhuis RA, Schütte PR. Resection rates and postoperative mortality in 7,899 patients with lung cancer. Eur Respir J 1996;9:7-10. [PubMed]
- Little AG, Gay EG, Gaspar LE, et al. National survey of non-small cell lung cancer in the United States: epidemiology, pathology and patterns of care. Lung Cancer 2007;57:253-60. [PubMed]
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6; discussion 2056. [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65 Suppl 3:iii1-27. [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [PubMed]
- Strand TE, Rostad H, Damhuis RA, et al. Risk factors for 30-day mortality after resection of lung cancer and prediction of their magnitude. Thorax 2007;62:991-7. [PubMed]
- Wada H, Nakamura T, Nakamoto K, et al. Thirty-day operative mortality for thoracotomy in lung cancer. J Thorac Cardiovasc Surg 1998;115:70-3. [PubMed]
- Powell HA, Tata LJ, Baldwin DR, et al. Early mortality after surgical resection for lung cancer: an analysis of the English National Lung cancer audit. Thorax 2013;68:826-34. [PubMed]
- Kozower BD, Sheng S, O’Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg 2010;90:875-81; discussion 881-3. [PubMed]
- Dillman RO, Zusman DR, McClure SE. Surgical resection and long-term survival for octogenarians who undergo surgery for non-small-cell lung cancer. Clin Lung Cancer 2009;10:130-4. [PubMed]
- Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 2007;25:5570-7. [PubMed]
- Falcoz PE, Conti M, Brouchet L, et al. The Thoracic Surgery Scoring System (Thoracoscore): risk model for in-hospital death in 15,183 patients requiring thoracic surgery. J Thorac Cardiovasc Surg 2007;133:325-32. [PubMed]
- Schipper PH, Diggs BS, Ungerleider RM, et al. The influence of surgeon specialty on outcomes in general thoracic surgery: a national sample 1996 to 2005. Ann Thorac Surg 2009;88:1566-72; discussion 1572-3. [PubMed]
- Farjah F, Flum DR, Varghese TK Jr, et al. Surgeon specialty and long-term survival after pulmonary resection for lung cancer. Ann Thorac Surg 2009;87:995-1004; discussion 1005-6. [PubMed]
- Cheung MC, Hamilton K, Sherman R, et al. Impact of teaching facility status and high-volume centers on outcomes for lung cancer resection: an examination of 13,469 surgical patients. Ann Surg Oncol 2009;16:3-13. [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [PubMed]
- Harpole DH Jr, DeCamp MM Jr, Daley J, et al. Prognostic models of thirty-day mortality and morbidity after major pulmonary resection. J Thorac Cardiovasc Surg 1999;117:969-79. [PubMed]
- Brunelli A, Varela G, Salati M, et al. Recalibration of the revised cardiac risk index in lung resection candidates. Ann Thorac Surg 2010;90:199-203. [PubMed]
- Cerfolio RJ, Minnich DJ, Bryant AS. General thoracic surgery is safe in patients taking clopidogrel (Plavix). J Thorac Cardiovasc Surg 2010;140:970-6. [PubMed]
- POISE Study Group, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008;371:1839-47. [PubMed]
- Poldermans D, Schouten O, Vidakovic R, et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V Pilot Study. J Am Coll Cardiol 2007;49:1763-9. [PubMed]
- Schouten O, van Kuijk JP, Flu WJ, et al. Long-term outcome of prophylactic coronary revascularization in cardiac high-risk patients undergoing major vascular surgery (from the randomized DECREASE-V Pilot Study). Am J Cardiol 2009;103:897-901. [PubMed]
- Brunelli A, Refai MA, Salati M, et al. Carbon monoxide lung diffusion capacity improves risk stratification in patients without airflow limitation: evidence for systematic measurement before lung resection. Eur J Cardiothorac Surg 2006;29:567-70. [PubMed]
- Ferguson MK, Siddique J, Karrison T. Modeling major lung resection outcomes using classification trees and multiple imputation techniques. Eur J Cardiothorac Surg 2008;34:1085-9. [PubMed]
- Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 2006;81:1830-7. [PubMed]
- Ferguson MK, Little L, Rizzo L, et al. Diffusing capacity predicts morbidity and mortality after pulmonary resection. J Thorac Cardiovasc Surg 1988;96:894-900. [PubMed]
- Pierce RJ, Copland JM, Sharpe K, et al. Preoperative risk evaluation for lung cancer resection: predicted postoperative product as a predictor of surgical mortality. Am J Respir Crit Care Med 1994;150:947-55. [PubMed]
- Ferguson MK, Vigneswaran WT. Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann Thorac Surg 2008;85:1158-64; discussion 1164-5. [PubMed]
- Linden PA, Bueno R, Colson YL, et al. Lung resection in patients with preoperative FEV1 < 35% predicted. Chest 2005;127:1984-90. [PubMed]
- Baldi S, Ruffini E, Harari S, et al. Does lobectomy for lung cancer in patients with chronic obstructive pulmonary disease affect lung function? A multicenter national study. J Thorac Cardiovasc Surg 2005;130:1616-22. [PubMed]
- Alam N, Park BJ, Wilton A, et al. Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg 2007;84:1085-91; discussion 1091. [PubMed]
- Brunelli A, Refai M, Salati M, et al. Predicted versus observed FEV1 and DLCO after major lung resection: a prospective evaluation at different postoperative periods. Ann Thorac Surg 2007;83:1134-9. [PubMed]
- Varela G, Brunelli A, Rocco G, et al. Measured FEV1 in the first postoperative day, and not ppoFEV1, is the best predictor of cardio-respiratory morbidity after lung resection. Eur J Cardiothorac Surg 2007;31:518-21. [PubMed]
- Win T, Jackson A, Groves AM, et al. Comparison of shuttle walk with measured peak oxygen consumption in patients with operable lung cancer. Thorax 2006;61:57-60. [PubMed]
- Brunelli A, Xiumé F, Refai M, et al. Peak oxygen consumption measured during the stair-climbing test in lung resection candidates. Respiration 2010;80:207-11. [PubMed]
- Brunelli A, Refai M, Xiumé F, et al. Performance at symptom-limited stair-climbing test is associated with increased cardiopulmonary complications, mortality, and costs after major lung resection. Ann Thorac Surg 2008;86:240-7; discussion 247-8. [PubMed]
- Kozower BD, Sheng S, O’Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg 2010;90:875-81; discussion 881-3. [PubMed]
- Brutsche MH, Spiliopoulos A, Bolliger CT, et al. Exercise capacity and extent of resection as predictors of surgical risk in lung cancer. Eur Respir J 2000;15:828-32. [PubMed]
- Loewen GM, Watson D, Kohman L, et al. Preoperative exercise Vo2 measurement for lung resection candidates: results of Cancer and Leukemia Group B Protocol 9238. J Thorac Oncol 2007;2:619-25. [PubMed]
- Brunelli A, Belardinelli R, Refai M, et al. Peak oxygen consumption during cardiopulmonary exercise test improves risk stratification in candidates to major lung resection. Chest 2009;135:1260-7. [PubMed]
- Bolliger CT, Jordan P, Solèr M, et al. Exercise capacity as a predictor of postoperative complications in lung resection candidates. Am J Respir Crit Care Med 1995;151:1472-80. [PubMed]
- Win T, Jackson A, Sharples L, et al. Cardiopulmonary exercise tests and lung cancer surgical outcome. Chest 2005;127:1159-65. [PubMed]
- Mason DP, Subramanian S, Nowicki ER, et al. Impact of smoking cessation before resection of lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database study. Ann Thorac Surg 2009;88:362-70; discussion 370-1. [PubMed]
- Lau KK, Martin-Ucar AE, Nakas A, et al. Lung cancer surgery in the breathless patient--the benefits of avoiding the gold standard. Eur J Cardiothorac Surg 2010;38:6-13. [PubMed]
- Choong CK, Meyers BF, Battafarano RJ, et al. Lung cancer resection combined with lung volume reduction in patients with severe emphysema. J Thorac Cardiovasc Surg 2004;127:1323-31. [PubMed]
- Riesenberg H, Lübbe AS. In-patient rehabilitation of lung cancer patients--a prospective study. Support Care Cancer 2010;18:877-82. [PubMed]


