Lingular segmentectomy and left lower lobectomy via unique bronchial dissection
Introduction
In general, bronchoplasty is a procedure performed on tumors located near the hilar area to achieve radical resection while preserving pulmonary function by avoiding pneumonectomy (1-5). Bronchoplasty requires a rather complicated technique that involves bronchial sutures after division of the bronchus and is associated with the risk of serious postoperative complications, including dehiscence of the bronchial sutures (6). We recently encountered a case of adhesion of the interlobar lymph node to the bronchus, which made conventional left lower lobectomy unfeasible. To avoid bronchoplasty, we successfully performed lingular segmentectomy and left lower lobectomy by devising a unique dissection line.
Case presentation
A 68-year-old male patient visited our center because of abnormalities found on chest radiography (Figure 1). He was a heavy smoker, smoking 1 pack a day for 40 years. His medical history included sarcoidosis, which had occurred 3 years previously and required regular visits at another hospital. Blood examination showed elevated levels of certain tumor markers, e.g., carcinoembryonic antigen (CEA) 34.3 ng/mL and sialyl Lewis X-1 (SLX) 170 U/mL.
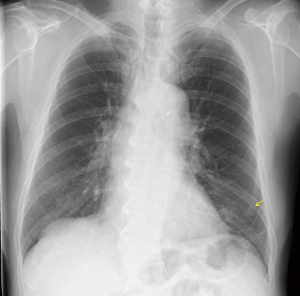
Granular shadows and thickening of the interlobular septum due to sarcoidosis, as well as a cyst in the left lower lobe, had been found 3 years previously. During follow-up, the cyst was clearly enlarged, and a nodule measuring 1.5 cm was present in the cyst, and the interlobar lymph node was swollen (Figure 2A,B). Fluorodeoxyglucose (FDG) positron emission tomography revealed accumulation of FDG in the intracystic nodule (SUVmax: 2.2) and interlobar lymph node (SUVmax: 10.5) (Figure 2C,D). These findings led to a diagnosis of lung cancer, cT1bN1M0, stage IIB, and it was decided that surgery would be implemented.

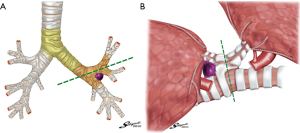
Initially, left lower lobectomy and lymph node dissection were planned, but it was found intraoperatively that the interlobar lymph node adhered to the bronchus in the bifurcation area between the upper and lower lobes, making bronchial dissection difficult. Because the enlargement of the lymph node was attributable to cancer metastasis, the plan was changed to include resection of the lingular segment to extirpate the lymph node en bloc. The pulmonary vein and artery in the lingular segment were ligated and dissected, and the intersegmental part of the lung was dissected and sutured with an automatic suturing device. A bronchial dissection line was drawn from the bifurcation between the superior division and lingular bronchi to the left main bronchus (Figures 3A,S1). The bronchus was held with a surgical stapler, and air supply to the superior division segment was confirmed. Then, the lingular segment and lower lobe bronchi were sutured and dissected en bloc to extirpate the specimen (Figures 3B,S1). The bronchial stump was reinforced by covering it with an intercostal muscle flap.

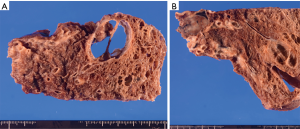
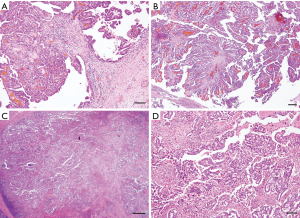
In the resected specimen, a 1.5-cm nodule in a cyst measuring 3.0 cm in diameter and interlobar lymph node swollen to 2.0 cm were macroscopically observed (Figure 4A,B). Histopathologically, the intracystic nodular cancer was revealed to be a papillary adenocarcinoma with polypoid expansion of atypical cells in the cyst. There were no malignant findings in the cystic wall in areas other than the area of the polypoid lesion (Figure 5A,B). Although the interlobar lymph node was filled with atypical cells, the capsule was intact, and no infiltration outside the lymph node was found (Figure 5C,D).
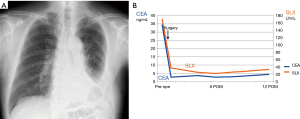
The patient is currently on oral tegafur and uracil as adjuvant therapy. There has been no recurrence to date 17 months after surgery, and the tumor markers have normalized (Figure 6A,B).
Discussion
The frequency of cavity formation in primary lung cancer is reported to be 2–16%, with squamous cell carcinoma and adenocarcinoma accounting for 45–63% and 30–53%, respectively (7). The mechanisms proposed for cavity formation include: (I) ischemic necrosis due to the occlusion of feeding vessels; (II) the check-valve mechanism of the conducting bronchus; (III) elastic traction by the surrounding lung tissue; (IV) tumor development in pre-existing bullae and (V) neoplastic cell autophagy (8-12). It is known that cancerous cavities often have a thick wall with an irregular inner surface (13-15). However, the cancer in our patient was unique in that it grew in a polypoid fashion toward the luminal side and that there was no cancer invasion in most parts of the inner surface of the cyst, with the cystic wall being thin. In addition, no malignant findings were noted in the cystic wall in the surrounding areas, suggesting that tumor development in the pre-existing bullae is the most likely mechanism of pathogenesis. Meanwhile, the following are cited from the literature as possible causes of lung cancer development in an emphysematous cystic wall (16-18): (I) scars of the cystic wall due to repeated infection; (II) squamous metaplasia of the epithelium constituting the cystic wall; and (III) insufficient ventilation in the cystic wall causes stagnation of various carcinogens.
Radiological differentiation between a cancer nodule in the cavity and a fungus ball-mycetoma is relatively straightforward because of the characteristic fungus ball-like appearance of pulmonary aspergillosis (19). In addition, the mobility of a fungus ball with a change in the patient’s position helps in the diagnosis (14). In the present case, chest computed tomography in the supine position revealed that an intracavitary tumor had grown from the ventral to the luminal side, and it failed to move with a change in position, suggesting that aspergilloma was unlikely.
Bronchoplasty is usually indicated for centrally located cancer to avoid pneumonectomy (3,4). A series of applications of extended sleeve lobectomy, which is a resection involving more than one lobe for oncologic or functional reasons, have been reported by a number of surgeons and have demonstrated feasibility (1,2,5). These resections include right upper and middle bilobectomy with or without segment 6, left upper lobectomy with segment 6, and left lower lobectomy with segments 4 and 5, which were then assigned to Okada types A, B, and C, respectively, according to the level of the bronchial anastomosis (2). When extended sleeve lobectomy is performed for the condition of the sort in our specific case, type C bronchoplasty is usually adopted. However, the angles of the bronchial surfaces to be anastomosed differ by more than 180° from the beginning, and therefore, the site of the bronchial suture becomes tensed, which incurs the risk of postoperative dehiscence (6). Because the continuity of the bronchus is broken, the risk of sputum retention and atelectasis also increases (6). In addition, a diameter match is required for sutures, so the procedure presumably would require a suturing technique with a high degree of difficulty (6). In our present case, there was lymph node adhesion, but there was seemingly no direct cancer invasion into the upper lobe bronchial wall. Considering these aspects, we performed bronchial dissection from the bifurcation between the superior division and lingular bronchi to the left main bronchus and successfully achieved en bloc resection of the cancer.
Based on our search of the English reports in the literature, the surgical technique we used does not seem to have been reported, although there might be underreporting. This procedure can be regarded as being the left-side version of the middle and lower lobectomy of the right lung and involves the drawing of a unique dissection line. This original and unique surgical procedure is supposed to be used in exceptional cases, unlike classical techniques of bronchoplasty, in that it does not require suturing, and it is useful in certain specific situations, with clinical benefit limited by the low frequency of such situations.
The whole length of the bronchial stump is long, so caution should be exercised to preserve blood flow in the bronchial stump during dissection. It is desirable to cover the stump with a muscle flap or fat pad to avoid a bronchial stump fistula. Meanwhile, this procedure may have a drawback in that poor ventilation is likely to occur in the superior division segment. The dissection line should be drawn with utmost caution so as not to cause bronchial stenosis, and a steady supply of air to the superior division segment should be confirmed by having the anesthesiologist send air just before firing the surgical stapler. In addition, bronchoscopic observation by the anesthesiologist may be of considerable help to confirm the patent airway to the superior division segment.
In conclusion, this surgical technique is an effective alternative to type C bronchoplasty in cases where resection of the left lower lobe and left lingular segment is necessary.
Acknowledgements
The authors greatly appreciate Yoshihiro Miyashita, Yumiko Kakizaki, and Toshiharu Tsutsui for their helpful scientific discussions. The authors also appreciate Shigeyuki Sakaguchi for drawing the illustrations in Figure 3.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for the publication of this manuscript and all accompanying images.
References
- Chida M, Minowa M, Miyoshi S, et al. Extended sleeve lobectomy for locally advanced lung cancer. Ann Thorac Surg 2009;87:900-5. [Crossref] [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Extended sleeve lobectomy for lung cancer: the avoidance of pneumonectomy. J Thorac Cardiovasc Surg 1999;118:710-3; discussion 713-4. [Crossref] [PubMed]
- Rendina EA, De Giacomo T, Venuta F, et al. Lung conservation techniques: bronchial sleeve resection and reconstruction of the pulmonary artery. Semin Surg Oncol 2000;18:165-72. [Crossref] [PubMed]
- Rendina EA, Venuta F, de Giacomo T, et al. Parenchymal sparing operations for bronchogenic carcinoma. Surg Clin North Am 2002;82:589-609. vii. [Crossref] [PubMed]
- Yamamoto K, Miyamoto Y, Ohsumi A, et al. Sleeve lung resection for lung cancer: analysis according to the type of procedure. J Thorac Cardiovasc Surg 2008;136:1349-56. [Crossref] [PubMed]
- Berthet JP, Paradela M, Jimenez MJ, et al. Extended sleeve lobectomy: one more step toward avoiding pneumonectomy in centrally located lung cancer. Ann Thorac Surg 2013;96:1988-97. [Crossref] [PubMed]
- Sugimoto Y, Semba H, Fujii S, et al. Clinical analysis of primary lung cancer with a thin-walled cavity to explain the mechanism of thin-walled cavity formation. Nihon Kokyuki Gakkai Zasshi 2007;45:460-4. [PubMed]
- Koizumi N, Akita S, Sakai K, et al. Classification of air density areas in CT-pathologic correlation of pulmonary adenocarcinoma. Radiat Med 1995;13:279-84. [PubMed]
- Weisbrod GL, Chamberlain D, Herman SJ. Cystic change (pseudocavitation) associated with bronchioloalveolar carcinoma: a report of four patients. J Thorac Imaging 1995;10:106-11. [Crossref] [PubMed]
- Weisbrod GL, Towers MJ, Chamberlain DW, et al. Thin-walled cystic lesions in bronchioalveolar carcinoma. Radiology 1992;185:401-5. [Crossref] [PubMed]
- Yoshida T, Harada T, Fuke S, et al. Lung adenocarcinoma presenting with enlarged and multiloculated cystic lesions over 2 years. Respir Care 2004;49:1522-4. [PubMed]
- Hirai S, Hamanaka Y, Mitsui N, et al. Primary lung cancer arising from the wall of a giant bulla. Ann Thorac Cardiovasc Surg 2005;11:109-13. [PubMed]
- Bandoh S, Fujita J, Fukunaga Y, et al. Cavitary lung cancer with an aspergilloma-like shadow. Lung Cancer 1999;26:195-8. [Crossref] [PubMed]
- Goto T, Kato R, Maeshima A, et al. Cavitary lung cancer with an aspergilloma-like shadow. J Thorac Oncol 2010;5:580-1. [Crossref] [PubMed]
- Goto T, Maeshima A, Oyamada Y, et al. Cavitary lung cancer lined with normal bronchial epithelium and cancer cells. J Cancer 2011;2:503-6. [Crossref] [PubMed]
- Kaneda M, Tarukawa T, Watanabe F, et al. Clinical features of primary lung cancer adjoining pulmonary bulla. Interact Cardiovasc Thorac Surg 2010;10:940-4. [Crossref] [PubMed]
- Stoloff IL, Kanofsky P, Magilner L. The risk of lung cancer in males with bullous disease of the lung. Arch Environ Health 1971;22:163-7. [Crossref] [PubMed]
- Wistuba II, Behrens C, Milchgrub S, et al. Sequential molecular abnormalities are involved in the multistage development of squamous cell lung carcinoma. Oncogene 1999;18:643-50. [Crossref] [PubMed]
- Solit RW, McKeown JJ Jr, Smullens S, et al. The surgical implications of intracavitary mycetomas (fungus balls). J Thorac Cardiovasc Surg 1971;62:411-22. [PubMed]
- Higuchi R, Nakagomi T, Goto T, et al. Surgical procedure of bronchial dissection. Asvide 2018;5:584. Available online: http://www.asvide.com/article/view/25559


