Video-assisted thoracoscopic versus open thoracotomy lobectomy: a Swedish nationwide cohort study
Introduction
Anatomic pulmonary lobectomy is the most common surgical procedure for operable lung cancer, and minimally invasive lobectomy by video-assisted thoracoscopic surgery (VATS) is increasingly used worldwide (1). The current evidence in support of VATS lobectomy over conventional open thoracotomy lobectomy is mainly based on observational non-randomized studies; national database analyses (2-6), institutional reports (7-9) and meta-analyses (10,11), with few exceptions (12). Most of the studies report results in favor of VATS lobectomy regarding perioperative complications, time to chest tube removal, hospital stay, pain and quality of life. Other studies have shown similar overall, cancer specific, and disease-free survival compared with patients undergoing thoracotomy lobectomy (13). Apart from the lack of high-level evidence, other factors could affect the widespread adoption. Minimally invasive surgery such as VATS lobectomy is a technically challenging procedure and surgeons may be reluctant to adopt the minimally invasive approach because of the initial learning curve that is involved. Our institution started a minimally invasive lobectomy program in 2012 as the first center in Sweden, and the purpose of this study was to investigate the safety of implementing minimally invasive lobectomies as the routine procedure for operable lung cancer, and to assess the long-term oncological efficacy because early stage lung cancer patients are referred for surgery with curative intent.
We performed a population based nationwide cohort study to investigate early and late clinical outcomes following the introduction of a minimally invasive thoracic surgery program. The aim was to investigate the early postoperative complications and long-term survival following minimally invasive VATS lobectomy compared to open thoracotomy lobectomy for early stage non-small cell lung cancer (NSCLC).
Methods
The study was approved by the regional Human Research Ethics Committee, Stockholm, Sweden (Dnr: 2014/129-31/1 and 2015/2338-32). The need for informed consent was waived by the committee.
Study design
This observational population-based cohort study followed the STROBE and RECORD guidelines for observational studies using routinely collected data (14,15).
Patients and outcomes measures
The national quality register for general thoracic surgery in Sweden (ThoR, http://www.ucr.uu.se/thor) was used to identify the study population. We included all patients registered in ThoR who underwent lobectomy for NSCLC between January 1, 2012 and December 31, 2015. ThoR was started in 2008, but a complete coverage of all eight thoracic surgery departments in Sweden was not achieved until 2013. During 2012, seven out of eight hospitals reported to ThoR. From 2013 and onward, all departments performing thoracic surgery reported to the register.
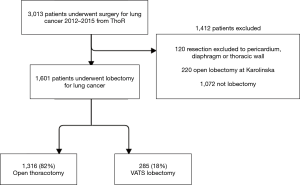
All patients who underwent minimally invasive lobectomy at Karolinska University Hospital were included in the VATS group. During the study period, a very limited number of patients (n=14) who underwent minimally invasive lobectomy at other hospitals in Sweden were excluded. We also excluded all patients who underwent open thoracotomy lobectomy at Karolinska University Hospital. Thus, the final study population consisted of the VATS group (minimally invasive lobectomy at Karolinska University Hospital) and a comparator group (open thoracotomy lobectomy performed at the other hospitals in Sweden who had not yet started minimally invasive lobectomy programs). All patients were operated on during the same time period (2012–2015). The patient inclusion flow chart is shown in Figure 1.
The primary outcome measure was all-cause mortality. Vital status was determined on April 15, 2017, by using the Swedish personal identity number (16) and the continuously updated Swedish population register (17). Follow-up was 100% complete. We also investigated a range of secondary outcome measures, mainly early postoperative complications that were available from the ThoR register.
Definitions
Comorbidity was defined as any major medical condition that required ongoing treatment or could influence prognosis, e.g., heart disease, diabetes, or history of stroke. Smoking status was divided into four categories: current, former, never, and unknown. Current smoker was defined as an active smoker or a person who had stopped smoking within 1 month of surgery. Former smoker was defined as a previous smoker who had stopped smoking more than 1 month before surgery. Never smoker was defined as a person who had never been an active smoker.
Operative technique
All patients in the VATS group were operated by one of three dedicated thoracic surgeons. We used a standardized three-port anterior approach as described by the Copenhagen group (18). Briefly, a 4–5 cm utility incision was made in the mid-axillary line between the 4th and 5th rib, and a 1 cm camera port was made lower down in the anterior axillary line at the level of the top of the diaphragm. Lastly, a 1.5-cm incision was made in the posterior axillary line at the same level of the camera port. The utility incision allowed for direct access to the lung hilum for dissection of vessels and bronchus, and to deal with complications or conversion to thoracotomy should an emergency situation arise (18). The surgeon and the assistant are positioned on the same side of the patient and use the same monitor, while the scrub nurse is positioned on the opposite side. We used a 30-degree rigid thoracoscope (Karl Storz & Co., Tuttlingen, Germany) in all VATS procedures.
Statistical analyses
Baseline characteristics were described with frequencies and percentages for categorical variables and mean and standard deviation for continuous variables. Person-time in days was counted from the date of operation until the date of death or the end of follow-up (April 15, 2017). The Kaplan-Meier estimator was used to calculate cumulative survival. Comparisons between open thoracotomy and minimally invasive lobectomy with respect to postoperative complications and long-term mortality were made using weighted logistic and Cox regression models where the weights were derived from propensity scores estimated using generalized boosted regression modeling. The following variables were used in the estimation of propensity scores: age, sex, body mass index, heart disease, diabetes, stroke, chronic kidney disease, other comorbidity, performance status, preoperative forced expiratory volume in one second, prior thoracic surgery, prior sternotomy, smoking status, adjuvant chemo- or radiotherapy, and pathological cancer stage. Balance between treatment groups was assessed by reporting standardized mean differences. A standardized difference of ≤0.1 was considered an ideal balance, and a standardized difference of ≤0.2 was regarded as acceptable balance. Statistical analyses were performed using Stata version 15.1 (StataCorp LP, College Station, TX, USA) and R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
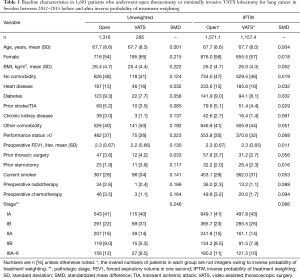
A total of 1,601 patients who underwent open lobectomy (n=1,316) or VATS lobectomy (n=285) for NSCLC between 2012 and 2015 in Sweden were analyzed (Figure 1). Patients who underwent procedures that extended beyond anatomical lobectomy or who underwent open lobectomy at Karolinska University Hospital were excluded. The mean age was 67.7 years in both groups but the proportion of women were higher in the VATS group. Comorbidities were in general well balanced, but the open thoracotomy group tended to have a higher proportion of patients with advanced pathologic cancer stage. After inverse probability of treatment weighting, all baseline characteristics were well balanced between the groups and the standardized mean difference were less than 0.1 in all variables. The baseline characteristics before and after inverse probability of treatment weighting are shown in Table 1.
Full table
Number of operations
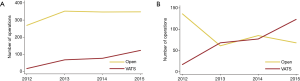
From 2012 through 2015, the number of VATS procedures increased while the open thoracotomy lobectomies remain stable (Figure 2). The number of open thoracotomy lobectomies at Karolinska University Hospital declined, in favor of an increase in the number of VATS procedures (Figure 2). During the last year of the study period, VATS lobectomies (n=123) were more common than open thoracotomy lobectomies (n=68).
Postoperative events and complications
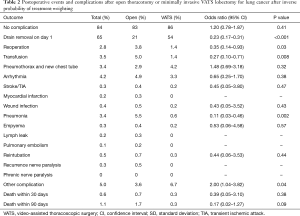
Postoperative events and complications were analyzed after inverse probability of treatment weighting and are shown in Table 2. The majority of patients (84%) did not have postoperative complications; 83% vs. 86% in the open thoracotomy and VATS group, respectively (P=0.41). The chest drains were removed on postoperative day 1 in 54% of the patients in the VATS group compared to 21% of the patients in the open thoracotomy group (P<0.001). The 30- and 90-day mortality was 0.7% vs. 0.3% (P=0.38) and 1.7% vs. 0.3% (P=0.09) in the open thoracotomy and VATS group, respectively. There were significantly more transfusions (5.0% vs. 1.4%, P=0.008) and pneumonia (5.5% vs. 0.6%, P=0.002) in the open thoracotomy compared to the VATS group. The number of days spent in hospital was significantly less in the VATS group compared to the open lobectomy group (median number of days: 4 vs. 6, P<0.001). A significantly larger proportion of patients in the VATS group was discharged to a rehabilitation facility instead of directly to their homes, compared to the open lobectomy group (63% vs. 30%, P<0.001).
Full table
Long-term survival
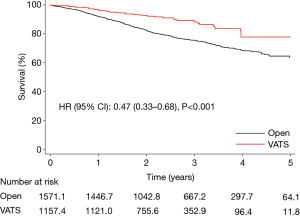
The median follow-up time was 2.6 and 2.3 years in the open thoracotomy and the VATS group, respectively. The overall survival at 1 and 5 years was 92% vs. 97% and 63% vs. 78% in the open thoracotomy and VATS group, respectively; HR (95% CI): 0.47 (0.33–0.68), P<0.001 (Figure 3). Results were consistent in a standard multivariable adjusted Cox regression model in the unweighted sample, and also in a “doubly robust” covariate adjusted weighted Cox regression model. The analysis was repeated in a subset of patients restricted to pathologic stage I–IIA, and the results were practically unchanged; HR (95% CI): 0.59 (0.39–0.88), P=0.009.
Discussion
The first reported Swedish series of VATS lobectomies included 30 patients and was published in 1998 (19). More than a decade later, a VATS lobectomy program was started at Karolinska University Hospital in 2012, and the VATS approach is currently the standard approach in the surgical treatment of NSCLC at our institution. The purpose of this study was to evaluate our initial experience of a dedicated shift in treatment strategy, and to ascertain the safety of an institution-wide implementation of a technically more demanding procedure.
The main findings of our study were that we showed a rapid increase in the volume of VATS lobectomies, and after only three years, the majority of the lobectomies were performed by VATS. We believe that the reasons explaining the rapid progress included the adoption of a well-organized and developed VATS program as described by the Copenhagen group (18). Also of importance was the involvement of three experienced and dedicated thoracic surgeons in a high-volume center that ensured a sufficient number of procedures per surgeon during a reasonably short time frame (1,20). Moreover, for an integrated team approach, we believe that it was important to use the same setting in all VATS lobectomies, i.e., the standardized three-port approach and the same surgical instruments and video-thoracoscope. Interestingly, McElnay et al. reported a dramatic increase in the VATS lobectomy rate following a formal training and adoption of the standardized anterior approach (21).
More than 80% of the patients in both groups in our study had no peri- or postoperative complications. Significantly more patients in the VATS group had their drains removed on postoperative day 1 and did not receive blood transfusions. Because the postoperative complication event rate was low, it was not possible to draw firm conclusions regarding the superiority of one method over the other, but the absence of a strong signal for a high complication rate in the VATS group was nevertheless reassuring. These findings were in line with previous reports (2,8,10,11,22). In our study, patients in the VATS group had a shorter hospital stay but were in a higher extent discharged to a rehabilitation facility instead of directly to their homes. This likely reflects differences in institutional policies and clinical care pathways at the hospitals, and it is probably not related to the surgical approach (6). However, in this context it is worth noting that one of the few randomized controlled trials in VATS surgery showed that VATS lobectomy was associated with less postoperative pain and better quality of life compared with anterolateral thoracotomy lobectomy (12). A particularly strong design feature of that study was that it was both patient and observer blinded.
In this study we compared outcomes in patients who underwent VATS lobectomy at one hospital with patients who underwent open lobectomy at the other seven hospitals performing thoracic surgery in Sweden. Patients who underwent open lobectomy at our institution were excluded from the study because they were deemed unsuitable as a control group as they had been considered inappropriate for VATS lobectomy. We also excluded a very small number of patients (n=14) who underwent VATS lobectomies at the other hospitals in Sweden during the study period. Because the distribution of baseline characteristics in the VATS group and the open lobectomy group was not balanced, we used a weighting procedure based on propensity score methods to achieve balance between the groups, and all outcome comparisons between the groups were made in the weighted sample. Although balance diagnostics suggested excellent balance after weighting, there may still remain unknown and unmeasured differences between the groups and all results must be interpreted with caution. The study was not designed to compare treatment effect and the main focus of the study was not evaluation of efficacy, but rather safety.
Similarly to a recent single-center study from Poland (7), we found better long-term survival in the VATS group. Two prior large studies found no significant differences in long-term survival (4,13) indicating an equally good oncological outcome and effectiveness of treatment compared with the open approach.
Study limitations
We lack data regarding the conversion rate because only the final surgical approach is noted in the register and not the intended or primary approach. This is an important limitation because it leads to the exclusion of patients who underwent conversion and may result in overly optimistic reporting of an implementation of a VATS lobectomy program. We also lack information regarding lymph node sampling. This is a limitation of our study because it has previously been shown that nodal upstaging has been lower after VATS lobectomy compared with open lobectomy for early stage NSCLC (3). Recent reports indicate safety of lymph node sampling during VATS lobectomy (5).
Conclusions
In this nationwide cohort study, we found that the establishment of a minimally invasive lobectomy program was possible within a short time frame, and without negatively affecting patient safety or the oncological efficacy of the treatment. Postoperative complications were generally infrequent, and long-term survival was better in the VATS group, although this must be interpreted cautiously and could be related to patient selection.
Acknowledgements
The authors thank the ThoR steering committee for providing data for this study.
Funding: This work was supported by the Swedish Heart-Lung Foundation (grant numbers 20160522, 20160525 to U Sartipy); the Mats Kleberg Foundation (2017-00096 to U Sartipy); Karolinska Institutet Foundations and Funds (2016fobi47721 to U Sartipy); Swedish Heart and Lung Association (E101/16 to U Sartipy); Åke Wiberg Foundation (M17-0089 to U Sartipy); Magnus Bergvall Foundation (2017-02054 to U Sartipy); the regional ALF agreement between Stockholm County Council and Karolinska Institutet (20160329 to U Sartipy); and a donation from Mr. Fredrik Lundberg (to A Franco-Cereceda).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the regional Human Research Ethics Committee, Stockholm, Sweden (Dnr: 2014/129-31/1 and 2015/2338-32). The need for informed consent was waived by the committee.
References
- Yan TD, Cao C, D'Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Licht PB, Jorgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943-9; discussion 949-50. [Crossref] [PubMed]
- Yang CJ, Kumar A, Klapper JA, et al. A National Analysis of Long-term Survival Following Thoracoscopic Versus Open Lobectomy for Stage I Non-small-cell Lung Cancer. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Gonfiotti A, Bertani A, Nosotti M, et al. Safety of lymphadenectomy during video-assisted thoracic surgery lobectomy: analysis from a national database†. Eur J Cardiothorac Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- von Meyenfeldt EM, Marres GMH, van Thiel E, et al. Variation in length of hospital stay after lung cancer surgery in the Netherlands†. Eur J Cardiothorac Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Dziedzic R, Marjanski T, Binczyk F, et al. Favourable outcomes in patients with early-stage non-small-cell lung cancer operated on by video-assisted thoracoscopic surgery: a propensity score-matched analysis†. Eur J Cardiothorac Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Laursen LO, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg 2016;49:870-5. [Crossref] [PubMed]
- Salati M, Brunelli A, Xiume F, et al. Video-assisted thoracic surgery lobectomy does not offer any functional recovery advantage in comparison to the open approach 3 months after the operation: a case matched analysis. Eur J Cardiothorac Surg 2017;51:1177-82. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Zhang Z, Zhang Y, Feng H, et al. Is video-assisted thoracic surgery lobectomy better than thoracotomy for early-stage non-small-cell lung cancer? A systematic review and meta-analysis. Eur J Cardiothorac Surg 2013;44:407-14. [Crossref] [PubMed]
- Bendixen M, Jorgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Paul S, Isaacs AJ, Treasure T, et al. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER-Medicare database. BMJ 2014;349:g5575. [Crossref] [PubMed]
- Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015;12. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. [Crossref] [PubMed]
- Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009;24:659-67. [Crossref] [PubMed]
- Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31:125-36. [Crossref] [PubMed]
- Hansen HJ, Petersen RH. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach - The Copenhagen experience. Ann Cardiothorac Surg 2012;1:70-6. [PubMed]
- Hermansson U, Konstantinov IE, Aren C. Video-assisted thoracic surgery (VATS) lobectomy: the initial Swedish experience. Semin Thorac Cardiovasc Surg 1998;10:285-90. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning curve associated with VATS lobectomy. Ann Cardiothorac Surg 2012;1:47-50. [PubMed]
- McElnay P, Casali G, Batchelor T, et al. Adopting a standardized anterior approach significantly increases video-assisted thoracoscopic surgery lobectomy rates. Eur J Cardiothorac Surg 2014;46:100-5. [Crossref] [PubMed]
- Ilonen IK, Rasanen JV, Knuuttila A, et al. Anatomic thoracoscopic lung resection for non-small cell lung cancer in stage I is associated with less morbidity and shorter hospitalization than thoracotomy. Acta Oncol 2011;50:1126-32. [Crossref] [PubMed]