Computed tomography-guided localization with laser angle guide for thoracic procedures
Introduction
Computed tomography (CT)-guided lung biopsy is an invasive but indispensable procedure associated with morbidity and mortality (1,2). To obtain sufficient specimen for diagnosis, core biopsy is often preferred to needle aspiration, which further increases the risk of complications (3). The Laser Angle Guide Assembly® (LAGA) was developed at our institution in an effort to improve the precision of punctures (4).
LAGA-assisted CT-guided biopsies were performed in 1,558 pulmonary lesions from 1999 to 2016. As an effective tool for lung cancer screening, low-dose CT became popular (5). The LAGA has also been applied to 101 CT-guided preoperative localizations for sub-centimeter tumors successfully in 2015 and 2016. For patients with lung malignancy deemed unsuitable for surgery, who had residual tumor after treatments, or with metastatic lung lesions, pulmonary tumor ablation is a choice for local disease control, though it also has high morbidity and mortality (6). We undertook pilot animal studies to understand the areas of potential risks (7). We introduced the LAGA to avoid injuring vital or risky structures, and to accurately target the planned locations. Since 2011, 265 pulmonary tumor ablations were performed successfully without any attempt abortion, major morbidity or mortality.
We describe herein our principal invention, the LAGA, and how our team utilizes this system to increase the precision of CT-guided pulmonary procedures.
Technique
The clinical application of this technique was approved by the Institutional Review Board of Chung Shan Medical University Hospital (#CS13221), and registered with the Food and Drug Administration, Taiwan.
Patients should be prepared with stable vital signs, adequate platelet counts, and normal blood coagulation tests. All the procedures were manipulated under local anesthesia.
Equipment
The LAGA comprised 2 major devices: a LAGA and laser levels (Figures 1,2) as described previously (4).
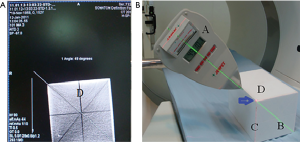
Calibration of LAGA
The LAGA should be calibrated every month or as needed as shown in Figure 1. The purpose of calibration is to synchronize the angle of LAGA and CT scan in the same plane of action.
The cubic model with radio-opaque lines is scanned by CT to acquire an angle of the selected line (Figure 1A). Then the portable green laser level is projected to the selected line on the cubic model (Figure 1B). The front index line of the LAGA follows the green line with its red laser point to the angle of the cubic model (blue arrow). An angle is shown on the LED screen of the LAGA, which is adjusted according to the angle acquired by the CT scan. The allowed error between the two angles is 0.1 degree after adjustment. At least two different lines should be examined.
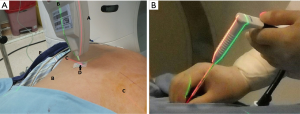
Manipulation of needle puncture (Figure 2, Table 1)

Full table
After positioning the patient firmly with a beanbag, a CT scan of the thorax is performed, an axial section with the target lesion is selected to plan the procedure. A skin surface needle puncture point (SSNPP) at the intercostal space with rib avoided is determined at the CT scan and the body of patient correspondingly on the selected section. A line from SSNPP to the target lesion on the CT scan is drawn with the angle and depth determined. The SSNPP at the body of the patient is aligned in the axial section of the stationary laser level. The angle of the LAGA is established according to the CT scan planned with its tip laser pointed to the SSNPP. The portable green laser level is projected to the front index line of the LAGA. The angle of the green laser will be identical to the angle planned on CT from the SSNPP aiming the lesion.
The LAGA is removed. A stopper is placed onto the puncture needle according to the pre-determined depth on CT scan. Precise puncturing of the needle is achieved along the intersection of the two laser lines to the lesion. The localization procedure was performed either by the Thoracic Surgeon or Chest Physician.
Applications
Core biopsies for lung tumor diagnosis instead of needle aspirations are preferred. After planning on CT scan to acquire the SSNPP, angle, and depth needed, a coaxial needle (15 G ×10 cm coaxial needle, TemnoTM, BD, USA) is advanced to the tumor border under the guidance of the LAGA system. Then the 16 G ×15 cm biopsy needle (TemnoTM, BD, USA) is fired to obtain tissues.
Pre-operative localizations of lung lesions are used with dye or a hookwire, which is selected according to the lesion depth from the pleura. The axial section, SSNPP, angle, and depths are planned according to the CT scans. If the lesion is less than 30 mm from the pleural, a 20 G, 89 mm spinal needle (Meditop, Thailand) will be introduced to the lesion under the guidance of the LAGA. Zero point one to 0.2 mL of 2.5% Patent Blue V (Guerbet, France) is injected (Figure 3A). The best dye visible depth is about 10 mm below the pleura. If the lesion is deeper than 30 mm, a hookwire (Hawkins™ II Breast Localization Needles. Argon, Texas, USA, 20 ga 7.5 cm) would usually be selected. To prevent the hookwire from dropping out, it is cut about 5–10 mm longer than the planned depth. Then the hookwire is placed to the planned position under the guidance of LAGA (Figure 3B). All lung resections should be performed within 8 hours from localization. The average procedure time was 19.5 minutes, standard deviation 8.4 minutes, for pre-operative localizations.
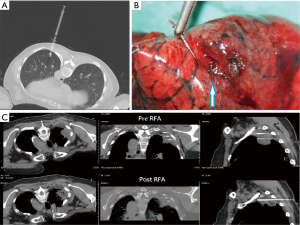
The cool-tipTM RFA with single or multiple electrodes (Medtronic, MN, USA) or a high-efficiency microwave ablation system equipped with a smart temperature energy control (AveCure® microwave ablation system, MedWaves, San Diego, CA, USA) are used for lung tumor ablations. The electrode/antenna should be inserted in the central core of the tumor. For tumors more than 3 cm, multiple electrode radiofrequency or microwave could be used (Figure 3C). Direct puncture of large vessels should be avoided. The esophagus should also be avoided (7). Following the above rules, a chest CT scan is done, an axial section is selected at the approximate center of the tumor, and the puncture plan is made. The ablation needle should be approached as close to the center of the tumor as possible under LAGA guide. The tumor is ablated according to the instruction of ablation systems.
Comments
To arrive at a target point in a 3-dimensional space human body, a 2-dimensional section with the target could potentially be used instead. From a start point selected in this section with a direction (angle) and a distance (depth), a specific target point could be reached. Nevertheless, most procedures were performed by ‘experienced hands’ without any precision angle navigation guide. In our system with the aid of CT scans, we first obtain a 2-dimensional axial section with the target lesion, followed by fitting the stationary axial laser to the corresponding body axial section with the target. Puncture plan is created on this selected CT with the SSNPP, angle, and depth determined. With the planned SSNPP at the surface of the body, the LAGA provides a reliable guide of the punctured angle allowing precise targeting on this 2-dimensional section.
The respiratory movement may influence the relative location of the tumor only when the lesion is near the diaphragm. Since the target lesion is so small and the patient is under clear conscious during the procedure, to prevent deviation from patient’s breathing movement, we do not ask patients to hold breath during the procedure because this maneuver could not be standardized among the patients. Instead, we catch the end-expiratory phase during both the CT scanning and puncture. Then the puncture and CT scan could be matched well.
Complication rates for CT-guided procedures were as high as 40% (1), with a 20–40% chance of pneumothorax (2,8). With this system, most of the procedures could be done in one shot. It decreases repeat punctures, avoids important structures, minimizes complications, saves times, as well as shortens learning curves especially for new comers or new procedures. This procedure was mature. We had a new surgeon who completed the localization well at the first attempt.
After the core biopsy, lung tumor ablation and preoperative localization could be introduced smoothly in our institution with near 2,000 procedures done (unpublished data). The radiation exposure for both staff and patients are reduced because repeated punctures and scans are not necessary, and real-time fluoroscopy is not needed. The time saved and the complications decreased render the LAGA system more cost-effective.
The LAGA system is the standard instrument for CT-guided invasive pulmonary procedures at our institution and may also be a great help for other medical staffs involved in this field, especially the new comers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yildirim E, Kirbas I, Harman A, et al. CT-guided cutting needle lung biopsy using modified coaxial technique: Factors effecting risk of complications. Eur J Radiol 2009;70:57-60. [Crossref] [PubMed]
- Hirose T, Mori K, Machida S, et al. Computed tomographic fluoroscopy-guided transthoracic needle biopsy for diagnosis of pulmonary nodules. Jpn J Clin Oncol 2000;30:259-62. [Crossref] [PubMed]
- Anderson JM, Murchison J, Patel D. CT-guided lung biopsy: Factors influencing diagnostic yield and complication rate. Clin Radiol 2003;58:791-7. [Crossref] [PubMed]
- Chan W. Laser angle guide assembly for computed tomography and method for operating the same. Available online: https://patents.google.com/patent/US20040117996
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Carrafiello G, Mangini M, Fontana F, et al. Complications of microwave and radiofrequency lung ablation: Personal experience and review of the literature. Radiol Med 2012;117:201-13. [Crossref] [PubMed]
- Lin FC, Tsai SC, Hsu JD, et al. Microwave ablation with permittivity feedback control in the lung of a porcine model: A safety test. Chung Shan Med J 2015;26:43-50.
- Simon CJ, Dupuy DE, Di Petrillo TA, et al. Pulmonary radiofrequency ablation: Long-term safety and efficacy in 153 patients. Radiology 2007;243:268-75. [Crossref] [PubMed]


