Congenital H-type tracheoesophageal fistula in adults
Congenital tracheoesophageal fistulas (TEFs) are rare. They are usually associated with esophageal atresia and therefore discovered and treated during the neonatal period. However, the H-type, which is not associated with esophageal atresia, may escape diagnosis in childhood and presents in the adult. This is the rarest type of congenital TEF, constituting only 4% of all congenital TEFs or about 1 in 100,000 live births. It was first described by Lamb in 1873 (1). The first report of a congenital tracheoesophageal fistula in an adult was in 1929 (2). The first successful transcervical repair was performed in 1939 by Imperatori (3). The oldest patient reported was 79 years old (4).
Because of constant aspiration, these patients suffer from recurrent pulmonary infection which can be fatal. Heightened alert regarding this condition is needed to allow early diagnosis and treatment. Only about 20 adult congenital H-type TEFs have been reported in the English literature (4-12). This report is adding three patients to the limited world experience. A good history and meticulous examination of the radiological examinations will help make the diagnosis.
Case #1
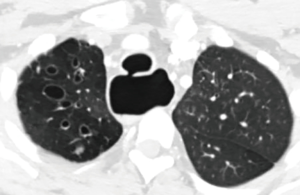
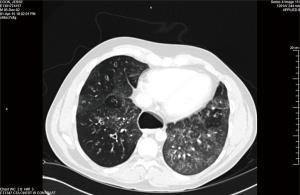
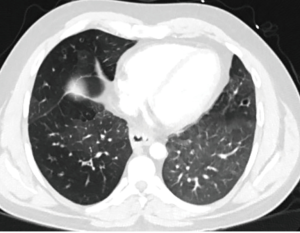
A 32-year-old man gave history that he had been throwing up when he tried to eat since he was a baby. He was diagnosed to have “severe reflux” when he was a baby and Nissen’s fundoplication together with gastrostomy tube was performed. The gastrostomy tube was removed when he was 4 years old but his coughing and regurgitation during meals persisted. He suffered from recurrent pulmonary infection and was repeatedly hospitalized. As a result, he also developed severe depression. In the 2 years prior to presentation, he was admitted to three other area hospitals repeatedly. He was seen by multiple pulmonary, gastrointestinal, and cardiac specialists. Multiple CT chest, barium swallow (Figure 1), and EGD were performed but the diagnosis was missed. CT scan demonstrated severe cystic bronchiectasis especially of the right upper lobe. The esophagus was grossly dilated, and air filled. In all of the above tests, the TEF was there but was missed by the examining physicians. He even had a stress test and a cardiac catheterization performed for the symptom of chest discomfort. In one hospital, the diagnosis of excessive tight Nissen’s fundoplication was made and he underwent laparoscopy, takedown of Nissen’s fundoplication, and had a partial wrap performed. His symptoms persisted after surgery and he was told that he might need an esophagectomy.

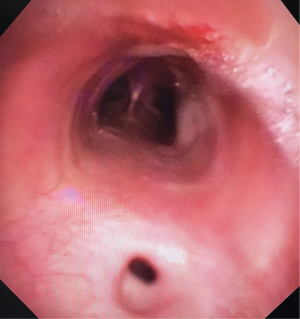
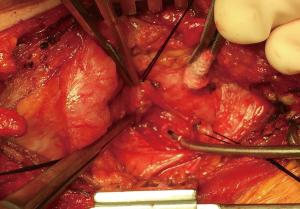
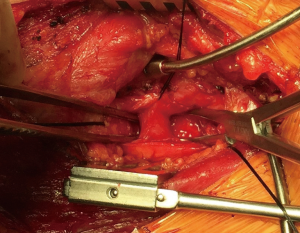
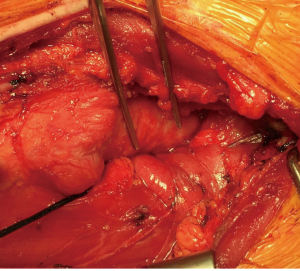
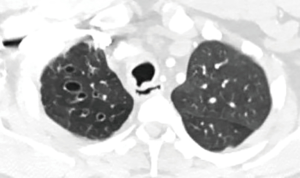
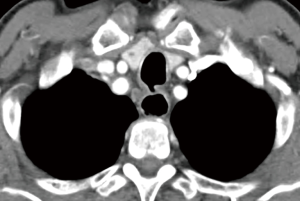
Then he presented to our hospital and our radiologist picked up the H-type TEF on the CT scan (Figures 2-4). Bronchoscopy (Figure 5) and esophagoscopy confirmed the diagnosis. The TEF was big enough for the bronchoscope to pass from the trachea into the esophagus (Figure 6). During EGD, I had to retroflex the scope in the dilated esophagus to visualize the TEF (Figure 7) because the TEF was slopping down from tracheal side to the esophageal side and the esophageal opening could not be seen when the scope was looking down from above. He was explored through a low cervical collar incision dissecting out the fistula from the right side since on CT scan, the TEF was more on the right side (Figure 2). The fistula was dissected out (Figure 8) and transected on the esophageal side (Figure 9) to allow suturing of the tracheal membranous wall defect with interrupted 4-0 Vicryl without tension. The esophageal opening was closed with 2 layers of interrupted 4-0 silk. The pedicled right sternohyoid muscle was used to interpose between the tracheal and esophageal repair (Figure 10). The swallow study on postoperative day #1 confirmed successful repair and he was discharged home 3 days after surgery eating a soft diet. Follow-up 2.5 years after surgery demonstrated persistent resolution of his symptom of coughing after meals. CT scan demonstrated marked improvement of his pulmonary inflammatory and bronchiectatic changes and the esophageal diameter is markedly decreased back to almost normal size (Figures 11,12). Barium swallow confirmed no recurrence of TEF. Esophageal peristalsis was unremarkable except for some intermittent breakup of the primary peristaltic wave and superimposition of a few tertiary esophageal contractions in the distal aspect of the thoracic esophagus consistent with mild distal esophageal dysmotility.


Case #2
A 49-year-old lady gave history of frequent respiratory infection and shortness of breath since she was a baby when she also underwent cardiac catheterization to rule out cardiac abnormality. Finally, at age 49, a barium swallow demonstrated congenital H-type TEF. She underwent repair through a left cervical approach using a technique similar to case #1. Her radiological pictures, bronchoscopic and EGD pictures were previously published in a textbook (15). She had complete recovery after repair.
Case #3
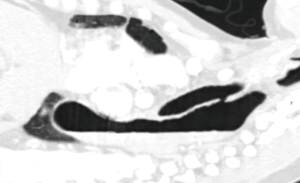
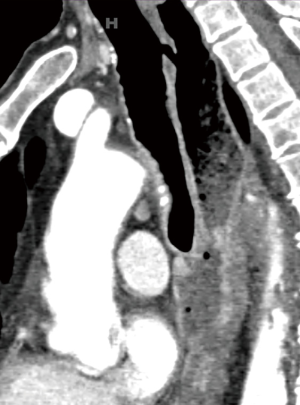
A 55-year-old man presented with dysphagia and 40 pounds weight loss. He gave history of coughing during swallowing for a long time and he could be drown by his own saliva. CT scan demonstrated right lower lobe pneumonia and distal esophageal cancer with liver metastases. However, there was also a congenital H-type TEF. Since the TEF is always more cephalad on the tracheal side, no one single axial image on the CT demonstrated the complete fistula resulting in the initial misinterpretation as a tracheal diverticulum (Figure 13). Sagittal reconstruction of the CT confirmed presence of TEF (Figure 14). Because both congenital H-type TEF and distal esophageal obstruction from cancer co-existed, his dilated esophagus was air-filled at the top and fluid filled in the middle and lower parts. Since recurrent aspiration is life threatening and surgical repair of the fistula is not complicated, he underwent cervical repair of the fistula. This time, after the TEF was isolated, it was easily stapled and divided with an endoscopic GIA stapler. Then interposition with the pedicled right sternohyoid muscle was performed. He had no more trouble with aspiration after repair.
Discussion
Congenital H-type TEFs are rare conditions but they are important because they result in constant aspiration and repeated pulmonary infection which is very debilitating and could be lethal. Most previously reported adult cases were isolated single case reports or at most report of two cases. This report of three cases is the largest series reported. The actual incidence must be higher than reported because many of them have escaped diagnosis and remain untreated. This report of three cases by a single author from a medium size city in the United States indicates that there are many more not diagnosed worldwide. Hopefully, more reports of these cases will increase awareness to allow these unfortunate patients to be diagnosed and treated.
Our case #1 reinforces the importance of systematically and carefully looking at every structure on any radiologic examination (CT and swallow study in this condition). If that had been done, the diagnosis would have been made much earlier.
One important clue to the diagnosis is an air filled dilated esophagus. This is because of the constant inflation of the esophagus by air forcing from the trachea through the fistula. When one sees an air filled dilated esophagus, one needs to carefully search for a TEF. On the other hand, a fluid filled dilated esophagus would be due to distal esophageal obstruction. In our case #3, both congenital H-type TEF and distal esophageal obstruction from cancer co-existed and his dilated esophagus was air-filled at the top and fluid filled in the middle and lower parts. This combination is first being reported here.
It is important to remember that the TEF is always more cephalad on the tracheal side. As a result, as illustrated by our case #3, a single axial view on the CT chest may not show up the entire fistula. It is important to exam also the sagittal view which will show the entire fistula very well. For the same reason, it was proposed that may be these TEF should be called N-type TEF instead of H-type TEF to more accurately reflect the anatomical oblique course of the fistula (6).
When the TEF is surgically divided and then sutured, Dr. Hermes C. Grillo taught me to divide the TEF on the esophageal side so that there is enough stump for tensionless repair on the membranous wall of the trachea. There is always no tension on the esophageal side for closure. Nowadays, these fistulas can be divided and closed with an endoscopic GIA stapler easily. On applying the stapler, one should similarly make sure that there is no tension on the membranous wall closure. Interposition of vascularized tissue between the two divided ends is important to prevent recurrence. Recurrent TEF presenting in adults after repair in childhood has been reported (12).
Can non-invasive or minimally techniques be applied to close congenital H-type TEFs? If the TEF is intrathoracic, VATS repair has been described. However, most congenital TEFs are in the cervical area. Can one perform an endoscopic procedure to obliterate the TEF? So far, I have not been able to come up with any sound procedure. It has been postulated to close the TEF with devices such as ASD closure devices. However, I can only imagine that the device, which is a foreign body, will erode through the tracheal and esophageal wall and cause a bigger TEF. Endoscopic thyroid resection has been developed but the technique is still controversial. Can we close congenital TEFs in a similar fashion? My main worry is the lack of good exposure risking damage to the recurrent nerve, trachea or esophagus. On the other hand, an open cervical incision is well tolerated by most patients and rapid recovery is the rule. Open surgery which gives good exposure and allows precise closure of the TEF with easy harvest of vascularised tissue to interpose between the two closed ends is still the preferred method, I think.
This is the first report with long term follow up that demonstrates that the dilated esophagus can reduce dramatically in size and the bronchiectasis can improve after repair of the TEF.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Informed Consent: Case #1 and #2 are lost to follow-up. Case #3 has passed away.
References
- Lamb DS. A fatal case of congenital tracheo-esophageal fistula. Phila Med Times 1873;3:705.
- Negus VE. Specimen: OEsophagus from a Middle-aged Man, showing a Congenital Opening into the Trachea. Proc R Soc Med 1929;22:527.
- Imperatori CJ. Congenital tracheoesophageal fistula without atresia of the esophagus: report of a case with plastic closure and cure. Arch Otorhinolaryngol 1939;30:352-9.
- Garand SA, Kareti LR, Dumont TM, et al. Thoracoscopic repair of tracheoesophageal fistula in a septuagenarian. Ann Thorac Surg 2006;81:1899-901. [Crossref] [PubMed]
- Zacharias J, Genc O, Goldstraw P. Congenital tracheoesophageal fistulas presenting in adults: presentation of two cases and a synopsis of the literature. J Thorac Cardiovasc Surg 2004;128:316-8. [Crossref] [PubMed]
- Black RJ. Congenital tracheo-oesophageal fistula in the adult. Thorax 1982;37:61-3. [Crossref] [PubMed]
- Acosta JL, Battersby JS. Congenital tracheoesophageal fistula in the adult. Ann Thorac Surg 1974;17:51-7. [Crossref] [PubMed]
- Azoulay D, Regnard JF, Magdeleinat P, et al. Congenital respiratory-esophageal fistula in the adult. Report of nine cases and review of the literature. J Thorac Cardiovasc Surg 1992;104:381-4. [PubMed]
- Newberry D, Sharma V, Reiff D, De Lorenzo F. A. "little cough" for 40 years. Lancet 1999;354:1174. [Crossref] [PubMed]
- Holman WL, Vaezy A, Postlethwait RW, et al. Surgical treatment of H-type tracheoesophageal fistula diagnosed in an adult. Ann Thorac Surg 1986;41:453-4. [Crossref] [PubMed]
- Hajjar WM, Iftikhar A, Al Nassar SA, et al. Congenital tracheoesophageal fistula: A rare and late presentation in adult patient. Ann Thorac Med 2012;7:48-50. [Crossref] [PubMed]
- Downey P, Middlesworth W, Bacchetta M, et al. Recurrent and congenital tracheoesophageal fistula in adults. Eur J Cardiothorac Surg 2017;52:1218-22. [Crossref] [PubMed]
- Suen HC. Barium swallow demonstrating barium went through a small TEF from the esophagus to the trachea and going up and down the trachea. Asvide 2018;5:556. Available online: http://www.asvide.com/article/view/25299
- Suen HC. The fiberoptic bronchoscope passed through the TEF into the esophagus and back. Asvide 2018;5:557. Available online: http://www.asvide.com/article/view/25300
- Grillo HC. Congenital and acquired tracheal lesions in children. In: Grillo HC. editor. Surgery of the Trachea and Bronchi. Hamilton/London: BC Decker, 2004:178.