Selective versus systematic lymph node dissection (other than sampling) for clinical N2-negative non-small cell lung cancer: a meta-analysis of observational studies
Introduction
Lung cancer is the most common cancer diagnosed and the leading cause of cancer-related deaths in China (1) and worldwide (2). The vast majority of cases are non-small cell lung cancer (NSCLC) where surgery plays a pivotal role among the associated therapeutic methods. As recommended by the guidelines (3,4), lobectomy with systematic lymph node dissection (LND) is regarded as the standard surgical treatment for early-stage NSCLC, which meant to provide accurate staging (5,6) detect occult metastasis (7) and improve survival (5,8,9). With the general acceptance of lung-cancer screening and the development of radiographic techniques, an increasing number of early-stage NSCLC patients can now be diagnosed. Therefore, minimally invasive approaches should be considered, such as video-assisted thoracic surgery, parenchyma-preserving resection and selective lymph node dissection (SLND) (10). The first two approaches have been well documented in the literature (11,12) and widely accepted in clinical practice while SLND remains controversial. Randomized trials have not demonstrated that LND had more survival benefit than sampling (7,13) while SLND is a totally different approach. SLND means specific lymph node stations are selected to dissect (14) according to the location, but sampling only makes a specimen of the relevant lymph node stations. Hence, whether SLND is comparable to LND and whether LND is favorable to prognosis stays unsolved.
SLND is now frequently mentioned because it is considered able to reduce operative time, blood loss, hospital stay, postoperative morbidity and mortality (15). Nowadays, advanced radiographic techniques such as positron emission computed tomography-computed tomography (PET-CT) can detect metastatic lymph nodes and provide accurate clinical stage. Therefore, the advantage of LND in comprehensive staging is weakened. Several retrospective studies (16-21) have revealed the rules of lobe-specific lymphatic drainage pattern. Meanwhile, some authors have put forward a concept of regional lymph nodes (22-25), which is similar to the idea of sentinel lymph nodes. They supposed that if regional lymph nodes are proved tumor-free, the rest should be preserved. On the basis of such findings (16-25), SLND may be applicable for early-stage NSCLC patients to minimize surgical trauma and provide clinical benefits.
Methods
Search strategy
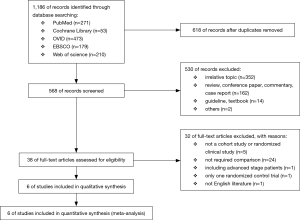
We searched several databases (PubMed, Cochrane Library, OVID, EBSCO and web of science) for pertinent literature from January 2006 to October 2016. In order to search comprehensively, we used the terms: (lung cancer OR lung carcinoma OR lung neoplasm OR nsclc OR NSCLC) AND (selective lymph node OR selected lymph node OR elective lymph node OR selective lymphadenectomy OR selected lymphadenectomy OR elective lymphadenectomy OR selective nodal OR selected nodal OR elective nodal OR selective mediastinal lymph node OR selected mediastinal lymph node OR elective mediastinal lymph node OR lobe specific) among title or abstract in each database. The specific process is available in the flow chart (Figure 1).
Inclusive criteria
Two reviewers screened the literature searched via the method above independently. Their judgments were based on the following inclusive criteria: (I) the comparison was between SLND and LND in surgical treatment of NSCLC; (II) all patients should be resectable clinical N2-negative NSCLC patients; (III) hazard ratio (HR) of overall survival (OS) could be calculated; (IV) the studies were published between 2006 and 2016.
Data extraction
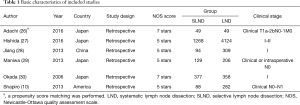
Two independent authors reviewed the full-text articles and extracted the data. Fundamental information of every study was summarized in accordance with the order: first author, year of publication, study design, population, lymphadenectomy strategy and clinical stage (Table 1). The primary endpoint was OS, and disease-free survival (DFS) was evaluated if available. In order to provide a comprehensive analysis of operative and postoperative conditions, we evaluated operative time, blood loss, postoperative morbidity and recurrence as well.
Full table
Quality assessment
The Newcastle-Ottawa Quality Assessment Scale was performed to estimate the bias risk for cohort studies in three aspects: selection, comparability and outcome. Maximum of 9 stars can be achieved regarded as minimum risk of bias and above 7 stars represents low risk of bias while below 4 stars means high risk of bias.
Data analysis
Survival (OS and DFS) was evaluated by HR, which was extracted along with associated 95% confidence interval (CI) if directly provided. The HR of the Hishida’s study was extracted from Cox regression model. Otherwise, the survival data were extracted from the Kaplan-Meier curve (except for Hishida’s study) by Engauge Digitizer software and HR was calculated by a specialized form designed by Tierney (31). For dichotomous data, risk ratio (RR) was calculated based on the number of events and total patients in each group. In addition, mean difference (MD) was calculated for continuous data derived from mean and standard deviation (SD) when presented in the article. If not, mean and SD were transformed from median and range by Hozo method (32).
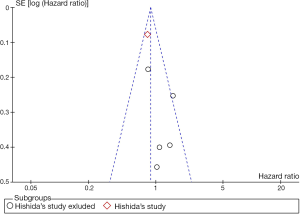
The χ2 test and I2 data were calculated to estimate the heterogeneity among the trials. Once upon Pheterogeneity <0.05 or I2 >50% occurred, heterogeneity was considered existing and the data would be analyzed following a random effects model. Otherwise, a fixed effect model would be employed. Moreover, publication bias was evaluated on the basis of funnel plot (Figure S1). P values above were two sided with 95% CI.
Results
Search results
A total of 1,186 potentially relevant articles were screened electronically, and the detailed reasons for exclusion were available in the flow chart (Figure 1). Six cohort studies were finally involved in this analysis. It is noteworthy that we excluded the only randomized controlled trial (RCT) we found in order to ensure the validity and reliability of our study. The basic characteristics of all studies are listed in Table 1 and some other vital variables are listed in Table S1.
Full table
Study interpretation
In all, 7,333 patients with clinical N2-negative NSCLC were included in this analysis and divided into two groups according to different strategies of lymph node dissection. A total of 2,005 patients were in the SLND group while the remaining 5,328 patients belonged to the LND group.
In the LND group, mediastinal lymph node stations #2R, #4R, #7, #8 and #9 should be dissected for the right lobes and mediastinal lymph node stations #4L, #5, #6, #7, #8 and #9 dissection is required for the left lobes. On both sides, N1 nodes are dissected as part of lung resection. In the SLND group, similar protocols were performed and the details are presented in Table S2. The mediastinal lymph node map is based on the International Association for the Study of Lung Cancer (IASLC) node map in the seventh edition of the TNM classification (33).
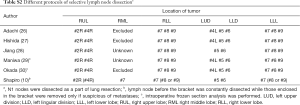
Full table
OS
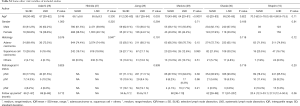
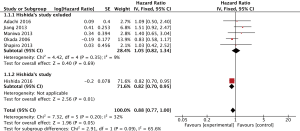
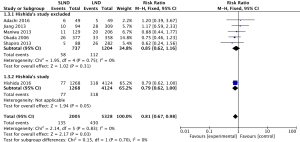
HRs were calculated from all six studies with 7,333 patients, and we regarded one of them (Hishida’s study) as a subgroup because of the sensitivity analysis. The influence of Hishida’s study on the pooled result could not be ignored (P value from 0.69 to 0.05), while the other studies did not have strong effects on the pooled result (Adachi’s study, P value from 0.04 to 0.05; Jiang’s study, P value from 0.01 to 0.05; Maniwa’s study, P value from 0.03 to 0.05; Okada’s study, P value from 0.09 to 0.05; Shapiro’s study, P value from 0.05 to 0.05). As shown in the forest plot, LND did not improve OS compared with SLND, no matter Hishida’s study was included (HR =0.88, 95% CI: 0.77–1.00, P=0.05, Figure 2) or not (HR =1.05, 95% CI: 0.82–1.34, P=0.69, Figure 2).
DFS
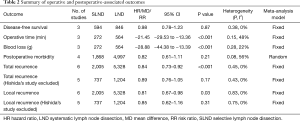
Data of 1,440 patients (594 in SLND group, 846 in LND group) from three studies were available to calculate HRs for DFS. In accordance with OS, there is no significant difference in DFS between LND and SLND (HR =0.98, 95% CI: 0.78–1.23, P=0.87, Table 2, Figure S2).
Full table

Operation associated outcomes
Three studies with 836 patients (272 in SLND, 564 in LND) were available to estimate operative time (min) and blood loss (g) by MD. In comparison with LND, SLND significantly reduced the operative time (MD =−21.45, 95% CI: −29.53 to −13.36], P<0.001, Table 2, Figure S3) and blood loss (MD =−28.88, 95% CI: −44.38 to −13.39, P<0.001, Table 2, Figure S4).


Postoperative morbidity
A total of 1,868 patients in SLND and 4,997 patients in LND were included to evaluate postoperative morbidity in terms of RR. The pooled results did not show significant difference (RR =0.82, 95% CI: 0.61–1.11, P=0.21, Table 2, Figure S5).

Recurrence
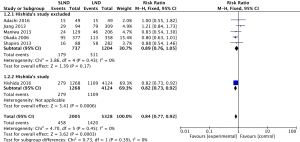
The data of recurrence were reported by six studies. After sensitivity analysis in total recurrence (Adachi’s study, P value from <0.001 to <0.001; Jiang’s study, P value from <0.001 to <0.001; Maniwa’s study, P value from <0.001 to <0.001; Okada’s study, P value from 0.02 to <0.001; Shapiro’s study, P value from <0.001 to <0.001, Hishida’s study, P value from 0.17 to <0.001) and local recurrence (Adachi’s study, P value from 0.02 to 0.03; Jiang’s study, P value from 0.02 to 0.03; Maniwa’s study, P value from 0.03 to 0.03; Okada’s study, P value from 0.06 to 0.03; Shapiro’s study, P value from 0.05 to 0.03, Hishida’s study, P value from 0.31 to 0.03), we set Hishida’s study as a subgroup. When Hishida’s study was included, both total recurrence (RR =0.84, 95% CI: 0.77–0.92, P<0.001, Table 2, Figure S6) and local recurrence (RR =0.81, 95% CI: 0.67–0.98, P=0.03, Table 2, Figure S7) showed significant difference under the comparison between SLND and LND. However, if Hishida’s study spared, there was no statistical difference (Table 2).
Discussion
During the process of literature search, only one published meta-analysis (34) has been found comparing the clinical outcomes between SLND and LND, but only three cohort studies were included (other than sampling). Besides, survival was the only analyzed outcome while the associated preoperative and postoperative outcomes were also included in our study.
Our meta-analysis demonstrated that SLND did not have negative influence on OS or DFS compared with LND. Instead, it would minimize the surgical trauma and has the potential to improve postoperative quality of life. On one hand, shorter operative time and less blood loss could be achieved in SLND, so that patients will suffer less from anesthesia and ischemia. On the other hand, the trend that SLND could decrease the postoperative morbidity should be noticed, which might contribute to quick recovery, shorter hospital stays and lower postoperative mortality rate. Moreover, although pooled results of recurrence were ambiguous because of the Hishida’s study, there was no evidence to suspect that SLND would increase the possibility of recurrence. Besides these clinical conclusions, it is also a strong support and encouragement for RCTs.
Our study has provided possible answers to some questions. Still, several problems remain unsolved. Whether SLND can reduce the postoperative mortality rate or shorten the hospital stay are not clear. In order to provide unambiguous answers, multi-institutional RCTs are expected to carry out in the future. Hence, it is crucial to formulate the specific definition and standard protocol of SLND because a conservative or radical option may reduce the efficacy of a clinical trial or cause adverse effect on patients. Moreover, although pathological types were comparable in each research included in our study, histological subtypes and intraoperative frozen section analysis should be taken into account in decisions on strategies of lymph node dissection. With the rapid development of radiographic field, some advanced techniques such as PET-CT should be performed before the surgery to provide accurate clinical stage. Meanwhile, the inclusion criteria of patients need to be cautiously considered.
Several limitations need to be acknowledged in our study. First and foremost, all the studies analyzed were cohort studies where selection bias and attrition bias were inevitable. For example, the patients’ selection and surgical approach were not randomized in each research included. And we suppose it is the reason why SLND showed decreased possibility of recurrence when Hishida’s study included. In fact, one eligible RCT (35), which indicates that the efficacy was similar in the SLND and LND group, has been screened out. Because we believe that it is inappropriate to combine an RCT with other retrospective studies and in order to ensure the validity and reliability of our study, we have to exclude it. In addition, most of the studies were carried out in Asia. Although the protocols of SLND were similar in different groups, no uniform criteria were formulated and the conversion from SLND group to LND group was not clearly described as well.
In conclusion, our meta-analysis indicated that SLND is an alternative to LND for clinical N2-negative NSCLC patients, which may even provide clinical benefits. However, more RCTs are expected to determine whether SLND is valid and practical to become a standard procedure of surgical treatment for early-stage NSCLC patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- De Leyn P, Lardinois D, Van Schil P, et al. European trends in preoperative and intraoperative nodal staging: ESTS guidelines. J Thorac Oncol 2007;2:357-61. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-e313S.
- Gajra A., Newman N, Gamble GP, et al. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol 2003;21:1029-34. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Complete Thoracic Mediastinal Lymphadenectomy Leads to a Higher Rate of Pathologically Proven N2 Disease in Patients With Non-Small Cell Lung Cancer. Ann Thorac Surg 2012;94:902-6. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non–small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Doddoli C, Aragon A, Barlesi F, et al. Does the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer? Eur J Cardiothorac Surg 2005;27:680-5. [Crossref] [PubMed]
- Lardinois D, Suter H, Hakki H, et al. Morbidity, Survival, and Site of Recurrence After Mediastinal Lymph-Node Dissection Versus Systematic Sampling After Complete Resection for Non-Small Cell Lung Cancer. Ann Thorac Surg 2005;80:268-74; discussion 274-5. [Crossref] [PubMed]
- Shapiro M, Kadakia S, Lim J, et al. Lobe-specific mediastinal nodal dissection is sufficient during lobectomy by video-assisted thoracic surgery or thoracotomy for early-stage lung cancer. Chest 2013;144:1615-21. [Crossref] [PubMed]
- Liu S, Wang R, Zhang Y, et al. Precise Diagnosis of Intraoperative Frozen Section Is an Effective Method to Guide Resection Strategy for Peripheral Small-Sized Lung Adenocarcinoma. J Clin Oncol 2016;34:307-13. [Crossref] [PubMed]
- Zhang Y, Sun Y, Wang R, et al. Meta-analysis of lobectomy, segmentectomy, and wedge resection for stage I non-small cell lung cancer. J Surg Oncol 2015;111:334-40. [Crossref] [PubMed]
- Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998;227:138-44. [Crossref] [PubMed]
- Han H, Chen H. Selective lymph node dissection in early-stage non-small cell lung cancer. J Thorac Dis 2017;9:2102-7. [Crossref] [PubMed]
- Ishiguro F, Matsuo K, Fukui T, et al. Effect of selective lymph node dissection based on patterns of lobe-specific lymph node metastases on patient outcome in patients with resectable non–small cell lung cancer: A large-scale retrospective cohort study applying a propensity score. J Thorac Cardiovasc Surg 2010;139:1001-6. [Crossref] [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Proposal for reasonable mediastinal lymphadenectomy in bronchogenic carcinomas: role of subcarinal nodes in selective dissection. J Thorac Cardiovasc Surg 1998;116:949-53. [Crossref] [PubMed]
- Aokage K, Yoshida J, Ishii G, et al. Subcarinal lymph node in upper lobe non-small cell lung cancer patients: Is selective lymph node dissection valid? Lung Cancer 2010;70:163-7. [Crossref] [PubMed]
- Shimada Y, Saji H, Kakihana M, et al. Retrospective Analysis of Nodal Spread Patterns According to Tumor Location in Pathological N2 Non-small Cell Lung Cancer. World J Surg 2012;36:2865-71. [Crossref] [PubMed]
- Turna A, Solak O, Kilicgun A, et al. Is Lobe-Specific Lymph Node Dissection Appropriate in Lung Cancer Patients Undergoing Routine Mediastinoscopy? Thorac Cardiovasc Surg 2007;55:112-9. [Crossref] [PubMed]
- Asamura H, Nakayama H, Kondo H, et al. Lobe-specific extent of systematic lymph node dissection for non–small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg 1999;117:1102-11. [Crossref] [PubMed]
- Haruki T, Aokage K, Miyoshi T, et al. Mediastinal Nodal Involvement in Patients with Clinical Stage I Non–Small-Cell Lung Cancer: Possibility of Rational Lymph Node Dissection. J Thorac Oncol 2015;10:930-6. [Crossref] [PubMed]
- Yoshimasu T, Miyoshi S, Oura S, et al. Limited mediastinal lymph node dissection for non-small cell lung cancer according to intraoperative histologic examinations. J Thorac Cardiovasc Surg 2005;130:433-7. [Crossref] [PubMed]
- Miyoshi S, Shien K, Toyooka S, et al. Validity of using lobe-specific regional lymph node stations to assist navigation during lymph node dissection in early stage non-small cell lung cancer patients. Surg Today 2014;44:2028-36. [Crossref] [PubMed]
- Kawano R, Hata E, Ikeda S, et al. Lobe-specific skip nodal metastasis in non-small cell lung cancer patients. Ann Thorac Cardiovasc Surg 2008;14:9-14. [PubMed]
- Shien K, Toyooka S, Soh J, et al. Clinicopathological characteristics and lymph node metastasis pathway of non-small-cell lung cancer located in the left lingular division. Interact Cardiovasc Thorac Surg 2015;20:791-6. [Crossref] [PubMed]
- Adachi H, Sakamaki K, Nishii T, et al. Lobe-Specific Lymph Node Dissection as a Standard Procedure in Surgery for Non-Small Cell Lung Cancer: A Propensity Score Matching Study. J Thorac Oncol 2017;12:85-93. [Crossref] [PubMed]
- Hishida T, Miyaoka E, Yokoi K, et al. Lobe-Specific Nodal Dissection for Clinical Stage I and II NSCLC: Japanese Multi-Institutional Retrospective Study Using a Propensity Score Analysis. J Thorac Oncol 2016;11:1529-37. [Crossref] [PubMed]
- Jiang W, Chen X, Xi J, et al. Selective mediastinal lymphadenectomy without intraoperative frozen section examinations for clinical stage I non-small-cell lung cancer: retrospective study of 403 cases. World J Surg 2013;37:392-7. [Crossref] [PubMed]
- Maniwa T, Okumura T, Isaka M, et al. Recurrence of mediastinal node cancer after lobe-specific systematic nodal dissection for non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;44:e59-64. [Crossref] [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Selective Mediastinal Lymphadenectomy for Clinico-Surgical Stage I Non–Small Cell Lung Cancer. Ann Thorac Surg 2006;81:1028-32. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Meng D, Zhou Z, Wang Y, et al. Lymphadenectomy for clinical early-stage non-small-cell lung cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2016;50:597-604. [Crossref] [PubMed]
- Ma W, Zhang ZJ, Li Y, et al. Comparison of lobe-specific mediastinal lymphadenectomy versus systematic mediastinal lymphadenectomy for clinical stage T(1)a N(0) M(0) non-small cell lung cancer. J Cancer Res Ther 2013;9 Suppl 2:S101-5. [Crossref] [PubMed]