Pneumothorax in teenagers: reducing recurrence through resection of superior segment of lower lobe
Introduction
During thoracoscopic surgery for pneumothorax, bullae or blebs are frequently found more often on the apical segment of the upper lung lobe than the superior segment of the lower lung lobe (lung subsegment “a” of segment 6, or S6a) (1). This means that if no obvious bullae are identified on S6a, the bullectomy is performed only on the apical bullae while the S6a is generally left alone. Despite the difference in frequency, however, both segments are notorious for the tendency to develop these abnormalities.
Through use of thoracoscopic magnified view during operations, it is now possible to see not only obvious apical bullae on the edge of S6a, but tiny non-ruptured blebs or several small suspicious lesions likely to be blebs in the future. In these cases, especially for young patients at our institution, we perform apical bullectomy and S6a resection concomitantly to remove suspicious lesions that had not yet ruptured at the time of the operation (given that written consent had been preoperatively obtained). We believe this improves the prognosis of our patients, but the benefits of this preemptive measure have not been widely reported. We decided to retrospectively investigate past cases to evaluate whether the S6a resection concomitant with apical bullectomy had prevented future pneumothorax recurrence in young patients 20 years of age or younger during their period of growth.
Methods
The study protocol was examined and approved by the Research Review Board at St. Mary’s Hospital on September 22, 2017 (approval number #17-0902). Written consent was obtained from all patients’ pre-operation.
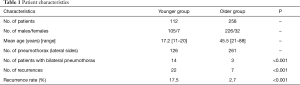
From April 2011 to September 2017, we performed 414 operations for pneumothorax at our institution (Table 1). Of those, 146 operations were performed on patients 20 years of age or younger (the younger group), and 268 operations were performed on patients 21 years of age or older (the older group).
Full table
The younger group was the focus of this study, and we conducted an investigation under the following conditions:
- If the same patient had both bilateral and contralateral pneumothoraces, each lateral pneumothorax was considered a different case;
- If the same patient experienced recurrent ipsilateral pneumothorax, the number of pneumothoraces after the first operation was considered to be the number of recurrences;
- We defined the recurrence rate as the ratio of the total number of recurrences to the total number of cases. To characterize the clinical features, both recurrence rates in the younger group and the older group were statistically compared;
- Regarding the effectiveness of S6a resection, two subgroups in the younger group—one involving patients having undergone S6a resection at the initial operation (the S6a group), and the other involving patients without S6a resection at the initial operation (the AB group)—were statistically compared in terms of recurrence rate; Incidentally, apical pleural reinforcement with an absorbable mesh sheet, which is mentioned in detail below, was not performed in the initial operation for the S6a group;
- To provide rationale for the S6a resections, all specimens obtained by the S6a resection in the initial operations were pathologically examined to determine whether the suspicious regions included actual bullae or bullous lesions.
Two or three ports were used during video-assisted thoracoscopic surgery (VATS) for spontaneous pneumothorax. For apical bullectomy, bullae were cut using scissors and sutured with an absorbent suture (PDS®II; Ethicon Inc., NJ, USA); however, if bullae were diffusely identified on the apex, staplers were employed. If non-ruptured bullae or several small suspicious lesions likely to be blebs in the future were identified on S6a, the edge of S6a was widely resected using staplers.
The numerical data to be compared were analyzed using the Chi-square test, employing the StatMateIII software program (ATMS Inc., Tokyo, Japan). A P value of <0.05 was considered to be statistically significant.
At our institution, an absorbable mesh sheet made from polyglycolic acid (such as NEOVEILTM; Gunze, Ayabe, Japan) to prevent recurrence was not often used in the initial operations for teenage patients because the mesh induces thick pleural adhesions on the apex, potentially complicating re-operations (1-4). Our views regarding the efficacy of the mesh sheet will be mentioned later in Discussion.
Incidentally, although we often performed apical bullectomy and pleural reinforcement using absorbable mesh, we did not include S6a resection unless obvious bullae or blebs were identified. In 2015, after experiencing several recurrent cases that caused us to reconsider our strategy, we decided to aggressively perform both apical bullectomy and S6a resection on teenage pneumothorax patients even when no obvious bullae were present on S6a.
Results
In the younger group of 105 males and 7 females (average 17.2 years; range, 11–20 years), 146 operations were performed on 126 pneumothoraces (lateral sides) and 14 of those 126 were bilaterally performed in the same patients. In the older group of 226 males and 32 females (average 45.5 years old; range, 21–88 years), 268 operations were performed for 261 pneumothoraces (lateral sides), and 7 of those 261 were bilaterally performed in the same patients.
In the younger group, pneumothorax reoccurred in 22 of 126 lateral sides, in 19 patients. In the older group, pneumothorax reoccurred in 7 of 261 lateral sides, in 7 patients. There was a significant difference between the younger group and the older group in terms of recurrence rate (17.5% vs. 2.7%, respectively; P<0.001).
In the younger group, S6a resection concomitant with apical bullectomy at the initial operation was performed in 56 of 126 lateral sides (S6a group), and apical bullectomy with apical pleura reinforcement using absorbable mesh was performed in 70 lateral side procedures (AB group) (Table 2). Of the former 56 lateral side procedures in the S6a group, recurrence occurred in 9; the responsible lesions of pneumothorax were on the apex in all 9 lateral sides. And of the latter 70 in the AB group, recurrence occurred in 13; the responsible lesions were on the apex in 5 and on S6a in 8. In total, recurrence rates of both groups were 16.1% in the S6a group and 18.6% in the AB group.

Full table
In 22 pneumothorax recurrence cases, the S6a recurrence rate was significantly low in the S6a group compared to the AB group (0.0% vs. 11.4%, P=0.025), while apical recurrence rate was high in the S6a group (16.1% vs. 7.1%, P=0.194; Table 3).

Full table
Pathologically, of 56 S6a specimens obtained at the initial operations in the S6a group, bullae or blebs were found in 24 specimens (42.9%), microscopic silent bullous lesions in 31 (55.4%), and no lesions in 1 (1.8%); at least one bullous lesion existed in 55 of 56 specimens (98.2%).
Discussion
It is commonly known that there are two types of spontaneous pneumothorax: primary pneumothorax, which often occurs in teenage males during a period of rapid growth, and secondary pneumothorax, which is mainly induced by smoking (excepting particular diseases, such as lymphangioleiomyomatosis or Birt-Hogg-Dube syndrome) and occurs in adults 25 years of age or older (5,6). However, since there is no clear-cut clinical borderline between the two types of pneumothorax, therapeutic tactics are often identical (7,8). But the clinical features of spontaneous pneumothorax in teenagers are completely different from those of secondary pneumothorax associated with smoking, as the bullae found in teenagers closely relate to physical growth (5). Although human beings do not undergo complete metamorphosis like insects, for the sake of analogy, a spontaneous pneumothorax in a teenager could be said to occur during the “pupal” stage. In fact, as our results show, the postoperative recurrence rate of teenage pneumothorax is significantly higher than that of adult patients, as if they were completely different diseases. A more appropriate course of action, then, would be to establish a distinct strategy for spontaneous pneumothorax in teenagers.
As mentioned above, although the recurrence rate is high in teenage pneumothorax, we (along with several others researchers) have reported significantly effective surgical procedures to prevent recurrence (1-4). Pasting absorbable polyglycolic acid mesh sheets on the lung surface employing fibrin glue, especially on the suture lines of apical bullectomy, is a very common procedure in Japan; at our institution we affix the sheet with a stitch on the bullectomy suture line without using fibrin glue (1-4). However, we are still faced with the puzzling reality that several patients who underwent a perfect apical bullectomy with the preventative procedure using absorbable mesh sheets still developed recurrent pneumothorax. In these cases, we often find a tiny air leakage from a small hole on the tip of S6a, which is barely visible under water, or a new bleb on S6a in those cases. The current study demonstrates that S6a is no less responsible than the apex in developing lesions that lead to pneumothorax recurrence, suggesting a blind spot in our clinical practice. In fact, according to our results, actual tiny bullae or microscopic bullous lesions existed in 98.2% of the S6a even at the time of the initial operations. However, we had not identified such potential risks for reoccurrence until analyzing the findings of this study.
We must also discuss the high recurrence rate of apical bullae post-surgery in this study. We had avoided using the absorbable mesh sheets during the initial operations for young patients as much as possible because the sheet induces thick adhesions between the lung and the parietal pleura (2,3,6). In fact, in our 9 cases with apical bullae recurrence, no absorbable mesh sheets were used because the adhesions might have complicated the second operation in case of recurrence, especially if the patients became smokers in the future (2). However, this approach may have inadvertently worked against the goal to reduce the recurrence rate during adolescence. The pleural adhesions and future smoking habit must remain elements to be carefully considered (6), but if the aim is to simply reduce the recurrence of pneumothorax during the patient’s youth, we recommend performing an apical bullectomy with absorbable mesh covering its suture line, concomitant with S6a resection as an initial operation for teenage pneumothorax patients. Theoretically, the apical mesh covering would improve the recurrence rate by 8.9% and S6a resection by 11.4%, and performing both procedures could reduce the recurrence rate from double digits to 7.1% at our institution.
Spontaneous pneumothorax during a period of growth is characterized by a high recurrence rate, and any measures to improve this rate should be carefully considered (5). The results of this study shed light on the fact that in addition to the apex, the S6a segment is often a breeding ground for bullous lesions. Harnessing this knowledge and adjusting the treatment strategy accordingly could potentially lead to an improved recurrence rate for spontaneous pneumothorax in teenagers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Research Review Board at St. Mary’s Hospital (approval number #17-0902) and written informed consent was obtained from all patients.
References
- Nakanishi K. An apical symphysial technique using a wide absorbable mesh placed on the apex for primary spontaneous pneumothorax. Surg Endosc 2009;23:2515-21. [Crossref] [PubMed]
- Muramatsu T, Nishii T, Takeshita S, et al. Preventing recurrence of spontaneous pneumothorax after thoracoscopic surgery: a review of recent results. Surg Today 2010;40:696-9. [Crossref] [PubMed]
- Obuchi T, Ueda M, Kawashita F, et al. A case of pneumothorax with Lymphangioleiomyomatosis undergoing pleurodesis using polyglycolic acid mesh. Jpn J Chest Surg 2005;19:628-31. [Crossref]
- Hirai K, Kawashima T, Takeuchi S, et al. Covering the staple line with a polyglycolic acid sheet after bullectomy for primary spontaneous pneumothorax prevents postoperative recurrent pneumothorax. J Thorac Dis 2015;7:1978-85. [PubMed]
- Tsuboshima K, Nagata M, Wakahara T, et al. Relationship between postoperative bulla neogenesis at the staple line and the resected lung volume in primary spontaneous pneumothorax. Gen Thorac Cardiovasc Surg 2015;63:572-5. [Crossref] [PubMed]
- Isaka M, Asai K, Urabe N. Surgery for secondary spontaneous pneumothorax: risk factors for recurrence and morbidity. Interact Cardiovasc Thorac Surg 2013;17:247-52. [Crossref] [PubMed]
- Hallifax RJ, Yousuf A, Jones HE, et al. Effectiveness of chemical pleurodesis in spontaneous pneumothorax recurrence prevention: a systematic review. Thorax 2017;72:1121-31. [Crossref] [PubMed]
- Uramoto H, Shimokawa H, Tanaka F. What factors predict recurrence of a spontaneous pneumothorax? J Cardiothorac Surg 2012;7:112. [Crossref] [PubMed]


