A tertiary care cancer center experience with carboplatin and pemetrexed in combination with pembrolizumab in comparison with carboplatin and pemetrexed alone in non-squamous non-small cell lung cancer
Introduction
Platinum-based chemotherapy is the standard first-line therapy for patients with non-small cell lung cancer (NSCLC) who do not carry any targetable “Driver” mutations (1). Carboplatin is the most commonly used platinum-based chemotherapy along with cisplatin in stage 4 NSCLC (2). Several studies have shown better prognosis (OS, better quality of life) in patients receiving platinum-based therapy compared to placebo (3-6). Platinum-based therapy has been used alone or in combination with other agents such as pemetrexed (a folate antimetabolite) and have shown better results (1,7).
Monoclonal antibodies (mAbs) against immune checkpoint inhibitors (ICIs) such as anti-PD-1, anti-CTLA-4, anti-PD-L1 and other immune co-stimulatory molecules (including anti-CD137) are being studied in solid tumors including NSCLC (8,9). Nivolumab was the first monoclonal antibody targeting PD-1 receptors investigated in solid tumors such as melanoma, NSCLC, renal cell carcinoma (9,10). Brahmer et al. showed improved median OS in patients receiving nivolumab vs. docetaxel (9.2 vs. 6 months, HR 0.59, P≤0.001) in patients who progressed on platinum doublet (11). In October 2015, FDA approved PD-L1 IHC 22C3 pharmDx assay by Merck in NSCLC (12). NSCLC patients who have PD-L1 expression >50% measured by this assay tend to have a better response to anti-PD-1 therapies (13,14). Atezolizumab (ICI targeting PD-L1) significantly improves OS compared to docetaxel in NSCLC (15). A KEYNOTE-024 phase III clinical trial compared pembrolizumab to the investigator’s choice cytotoxic chemotherapy as first-line therapy for patients with advanced NSCLC with a PD-L1 expression score of >50%. In this study, the median progression-free survival (PFS) was 10.3 months in pembrolizumab group vs. 6 months in the chemotherapy group (P<0.001, HR =0.50). Objective response rate (ORR) was also higher in the pembrolizumab group (44% vs. 27.8%) (16).
Most of the combination regimens in NSCLC are limited to two drugs. However, some studies have demonstrated the benefit of more than two drugs combinations (17,18). Bevacizumab (mAb against vascular endothelial growth factor) has been used in combination with paclitaxel and carboplatin (17) as well as with gemcitabine and cisplatin (18). These studies have shown positive results. Studies have shown that the chemotherapies also induce the immunity against the tumor cells via various mechanisms (19,20). Chemotherapies induce PD-L1 expression in the tumor cells that can be the target of mAbs against these ligands (21,22). To explore the synergistic effects of the chemotherapies and the anti-PD-1 mAbs, a phase I clinical trial combined pembrolizumab with carboplatin/pemetrexed and showed better outcomes (ORR 71%, median PFS of 10.2 months) (23). Cohort G1 of a phase 2 open-label KEYNOTE-021 study compared carboplatin/pemetrexed to carboplatin/pemetrexed/pembrolizumab. ORR was higher in the chemotherapy/pembrolizumab cohort (55% vs. 29%). Less proportion of patients experienced progression in chemotherapy/pembrolizumab group (29% vs. 50%). Patients with brain metastasis were excluded from this study (24). A subsequent phase 3, double-blind clinical trial (the KEYNOTE-189 study) showed improved OS (HR =0.49, P<0.001), median PFS (8.8 vs. 4.9, HR =0.52, P<0.001), and ORR (47.6% vs. 18.9%, P<0.001). Patients with brain metastases were included in this study (25).
In May 2017, after the FDA approval of the combination carboplatin/pemetrexed/pembrolizumab therapy in non-squamous NSCLC patients (26), we started using this combination at our institution. Patients who had brain metastases were also treated with this combination. Our retrospective study aims to evaluate the efficacy [ORR, disease control rate (DCR), PFS] of this combination therapy in our patient population with and without brain metastasis and compare these results with that of the Cohort G1 of the KEYNOTE-021 and KEYNOTE-189 studies.
Methods
This is a retrospective chart review study conducted at Norris Cotton Cancer Center and Dartmouth-Hitchcock Medical Center (DHMC). DHMC coding department was contacted to identify patients who have been diagnosed with advanced staged NSCLC. Patients older than 18 years of age diagnosed with non-squamous NSCLC since January 1st, 2016 till December 15th, 2017 who have received FDA approved carboplatin/pemetrexed (Cohort A) or carboplatin/pemetrexed/pembrolizumab (Cohort B) were selected. Patients with a history of prior chemotherapy, prior history of autoimmune disease or another malignancy were not excluded from this study. Exclusion criteria were the patients <18 years of age or the patients who have received ICIs before the treatment in any of the cohorts. IRB approval was obtained for this retrospective study.
Electronic chart review was performed to obtain the demographic, clinical and treatment data. In our patients, PD-L1 expression was measured by FDA approved PD-L1 expression assay with 22C3 anti-PD-L1 by Merck. Best radiographic response, i.e., complete remission (CR), progressive disease (PD), partial response (PR) and stable disease (SD) and the time to achieve the best response was recorded using RECIST criteria V 1.1 (27). CR was defined as radiographic disappearance of all target lesions, PR was defined as 30% decrease in the target lesions, SD was defined as no significant increase or decrease in the size of the target lesions and the PD was defined as appearance of the new lesions or the increase in the size of the known lesions (20% or more) (27). ORR was defined as a percentage of patients achieving PR and CR. DCR was defined as a percentage of patients achieving CR, PR, and SD. Time to best response was defined as the time from treatment date till the date of first documented response. OS was calculated from the initiation of the therapy till the last follow-up date (12/31/2017) in case patient is alive or until the time of death. PFS is defined as no objective worsening of the disease while the patient is on therapy, calculated from the date of therapy initiation till the last follow-up date (12/31/2017), date of progression of disease or the time of death. Data were also stratified based on the presence of brain metastases before initiation of the treatment.
Independent variables were the therapies, age, and sex. Dependent variables included best response, PFS, ORR, DCR, side effects of the therapy. Outcomes measured and compared: PFS, DCR, and ORR. ORR is the primary endpoint. PFS, DCR are the secondary endpoints.
Statistical analysis
Due to anticipated small sample size, no power analysis was performed, and non-probability convenience sampling was done. Summary measures of continuous data such as age at diagnosis, laboratory data, OS, PFS, mean, median, standard deviation (SD), inter-quartile range were calculated. Histograms and qq-plots of continuous endpoints were used to evaluate distributional assumptions. To evaluate the OS and PFS with 95% CI, Kaplan-Meier method and the log-rank test were applied. However, to take into account the other potential variables affecting the survival and progression, Cox regression was applied to calculate the hazard ratio. Chi-square and Fisher exact tests were applied to compare the categorical variables and calculate the P value. T-tests were applied to analyze the continuous variables and to calculate the P values. STATA V. 14.2 was used to perform statistical analysis.
Results
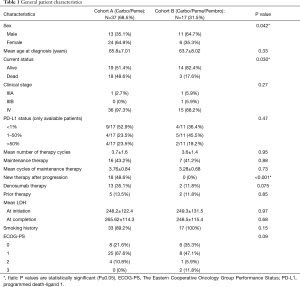
A total of 54 patients met the inclusion criteria. Twenty four (44.4%) patients were male, and 30 (55.6%) patients were female. Mean age at diagnosis of advanced stage NSCLC is 65.14±7.3 years. Overall 21 (38.9%) patients passed away by the time of last follow-up (12/31/2017). Forty-five (83.3%) patients had well-differentiated adenocarcinoma, and 9 (16.7%) patients have poorly differentiated adenocarcinoma. At the time of diagnosis, 51 (94.4%) patients were stage IV, 2 patients (3.7%) had stage IIIA disease and 1 (1.8%) patient had stage IIIB disease. Two patients received chemo-radiation as initial therapy, and 1 patient had lobectomy and then adjuvant chemotherapy before progressing into stage IV disease.
Cohort A (carboplatin + pemetrexed) included 37 (68.5%) patients and cohort B (carboplatin + pemetrexed + pembrolizumab) had 17 (31.5%) patients. Cohort A had more female patients (64.9% vs. 35.3%, P=0.042). Median follow-up time in cohort A was significantly higher than that of cohort B (12.85 vs. 4.99 months, P<0.001). Mean age at diagnosis in each cohort was 65.8±7.01 and 63.7±8.02 respectively (P=0.33). A higher proportion of patients died in cohort A (48.6% vs. 17.6%, P=0.030). PD-L1 expression (measured by Merck anti-PD-L1 expression assay) was available in only 28 (51.8%) patients due to lack of availability of the adequate tissue. Cohort A had slightly higher proportion of patients (23.5% vs. 18.1%) with PD-L1 expression >50%. More patients in cohort B were smokers (100% vs. 89.2%, P=0.15). Mean number of cycles for the combination therapy were similar between both groups (3.7 vs. 3.6, P=0.95). In cohort A, 16 (43.2%) patients received maintenance pemetrexed after finishing the induction with carboplatin + pemetrexed. In cohort B, 7 (41.2%) patients received maintenance with pemetrexed plus pembrolizumab after finishing induction with carboplatin, pemetrexed plus pembrolizumab (P=0.88). Six (16.2%) patients had received prior therapy before switching to combination therapy in cohort A compared to 2 (11.8%) patients in cohort B (P=0.7). One of these six patients from cohort A received prior chemo-radiation as adjuvant therapy and remaining five patients received chemotherapy before receiving this combination therapy. One of these two patients from cohort B received induction chemo-radiation, and one patient received adjuvant chemotherapy after undergoing lobectomy. Eighteen (48.6%) patients in cohort A were switched to new therapy after progression on combination therapy, and seventeen of these eighteen patients were switched to ICI. None of the patients in cohort B was switched to new therapy by the last follow up (P<0.001) (Table 1).
Full table
Pre-treatment mean number of metastatic sites involved were the same between both cohorts (3.6±1.2 vs. 3.8±2.4, P=0.56). Brain, liver, adrenal glands, pleura and the vertebral bodies were the most commonly involved sites. Pre-treatment distribution of the skeletal and brain metastasis was similar between both cohorts (51.4% vs. 53%, P=0.91 and 32.4% vs. 35.3%, P=0.83), whereas slightly higher proportion of patients in cohort B had metastatic liver involvement (29.4% vs. 18.9%, P=0.38). Post-treatment, cohort B had considerably less number of metastatic sites involvement compared to cohort A (B: 1.3±2.2 vs. A: 2.5±2.12, P=0.06) (Table 2).

Full table
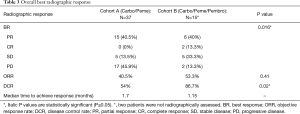
The difference in best response between both cohorts was significantly different (P=0.016). In two patients from cohort B, we were not able to assess radiographic response. Among 15 patients with evaluable radiographic response in cohort B, 6 (40%) patients achieved PR vs. 15 (40.5%) patients out of 37 patients in cohort A. Two (13.3%) patients achieved CR in cohort B vs. none in cohort A. Higher proportion of patients had PD in cohort A [17 (45.9%) vs. 2 (13.3%)]. ORR was higher in cohort B (53.3% vs. 40.5%, P=0.41). DCR was significantly higher in cohort B (86.7% vs. 54%, P=0.02). Moreover, the median time to achieve best response was shorter in cohort B as well (1.15 vs. 1.7 months) (Table 3).
Full table
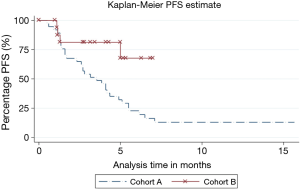
Significantly higher proportion of patients progressed in cohort A [31 (83.8%) vs. 4 (23.5%), P<0.001]. PFS was significantly different between both cohorts (P=0.009, HR 0.22, 95% CI =0.073–0.69) (Figure 1). The median PFS in cohort A was 3.55 months and was not reached in cohort B.
Brain metastases
Similar proportion of patients had brain metastases in each cohort [12 (32.4%) vs. 6 (35.3%), P=0.83]. Significantly higher proportion of patients were pre-treated with SRS for brain metastases in cohort A [10 (83.3%) vs. 1 (16.7%), P=0.013]. The proportion of patients receiving WBRT was not significantly different between both cohorts (41.7% vs. 33.3% respectively, P=0.85). All pretreated patients were symptomatic from brain metastasis. Response was not evaluable in one patient from cohort B. Systemic ORR was higher in cohort B (80% vs. 58.3%, P=0.75). CNS ORR was also higher in cohort B (80% vs. 41.7%, P=0.14). Brain lesions of all patients in cohort B showed significant CNS response. Two patients in cohort A only had systemic response. No significant difference in DCR was observed between both cohorts either (75% vs. 80% respectively, P=0.68). However, time to achieve best response was shorter in cohort B (1.1 vs. 1.67 months) (Table 4). Only 2 (16.7%) patients in cohort A and 3 (50%) patients in cohort B were not pre-treated before the combination therapy (asymptomatic). Of the non-pre-treated patients, 1 of 2 (50%) patients had PR in each cohort. The response was not assessed in one patient from cohort B.

Full table
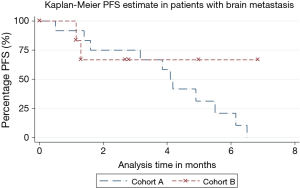
Significantly higher proportion of patients with brain metastases progressed in cohort A [11 (91.7%) vs. 2 (33.3%), P=0.009]. However, PFS was not statistically different (P=0.6, HR =0.67, 95% CI =0.11–3.9). Median PFS was not reached in cohort B and was 4.1 months in cohort A (Figure 2). Of the 11 patients with brain metastases from cohort A who progressed on the combination therapy, 4 (36.4%) patients experienced worsening of the previously known brain lesions or appearance of new lesions. Remaining 7 (63.7%) patients had systemic progression. None of the two patients who progressed in cohort B experienced new or worsening brain metastasis. One of these patients had progression of his systemic disease, and the other patient died before response assessment.
Discussion
FDA granted accelerated approval to a combination of pembrolizumab/carboplatin/pemetrexed as first line treatment for patients with advanced non-squamous non-small cell lung cancer in May 2017 (26). This approval was based on the results of the KEYNOTE-021 study (24) that showed ORR of 55% in pembrolizumab plus chemotherapy group vs. 29% in chemotherapy-only group (P=0.0016). The median PFS was also significantly longer in pembrolizumab plus chemotherapy group (P=0.01, HR =0.53 (95% CI: 0.31–0.91) (24). In earlier phase I study, the ORR was 57% in pembrolizumab plus chemotherapy group, and the median PFS was 10 months (23). Rizvi et al. investigated the anti-PD-1, nivolumab with different combinations of chemotherapies in NSCLC and found promising results (28). However, in that study, Bristol-Myers Squibb’s (BMS) PD-L1 IHC 28-8 pharmDx qualitative assay was used for PD-L1 expression which is not FDA approved yet. The ORR was 33%, 47%, 47% and 43% in patients with nivolumab and different combinations of doublet chemotherapies (28). Based on these positive results and FDA approval, we adopted the use of pembrolizumab with the doublet chemotherapy at our institution, and the early results have been encouraging. We also used this combination in patients with brain metastases who were excluded from the KEYNOTE-021 study. Pembrolizumab have been shown to be safe and effective in NSCLC with brain metastases (29). The documented safety and activity of monotherapy were our rationale for treating such patients with this combination. A recently concluded phase 3 KEYNOTE-189 trial also included asymptomatic or previously treated patients with brain metastasis. The included patients must be clinically stable for at least 2 weeks without any enlarging or new metastases and should not be on steroids for at least 3 weeks before the start of therapy. Patients who had asymptomatic brain metastases were also included (25). In our study, we also included patients with brain metastases who had received treatment (SRS, WBRT) before starting the combination therapy.
Due to the relatively recent approval of this regimen, the median follow-up time in our study was significantly shorter in cohort B (4.99 vs. 12.85 months, P<0.001). The median follow- up time in the KEYNOTE-021 study was 10.6 months and 10.5 months in KEYNOTE-189 study (24,25). The ORR in our study was almost similar to these studies (53.3% in our study vs. 55% in KEYNOTE-021 and 47.6% in KEYNOTE-189) (24,25), however ORR was not significantly different in our study (53.3% vs. 40.5%, P=0.41) likely due to small sample size. Further, patients from cohort A did relatively well vs. the patients from KEYNOTE-021 and KEYNOTE-189 studies (ORR of 40.5% vs. 29% vs. 18.9%). The greatest benefit of the pembrolizumab and the chemotherapy was observed in achieving disease control as the DCR was significantly higher in patients from cohort B (86.7% vs. 54%, P=0.02). In the KEYNOTE-021 study, DCR was 88% in pembrolizumab and chemotherapy group vs. 70% in chemotherapy-only group (24). In the KEYNOTE-189, DCR was 84.6% in pembrolizumab and chemotherapy group and 70.5% in the placebo and chemotherapy group (25). We did not measure the mean duration of the overall response due to a short median follow-up time. These findings suggest that the pembrolizumab plus chemotherapy not only achieve disease response but also stabilize the disease in advance stages more effectively compared to the chemotherapy alone.
In our study, PFS is significantly different between both cohorts (P=0.009, HR 0.22, 95% CI: 0.07–0.69), but the median PFS in cohort B was not reached. The median PFS was significantly prolonged in both the KEYNOTE-021 and the KEYNOTE-189 studies in patients receiving pembrolizumab-chemotherapy combinations [(13.0 vs. 8.9 months) and (8.8 vs. 4.9 months) respectively] (24,25). In our study, the median PFS for cohort A was just 3.55 months though, likely due to higher incidence of event (progression) rate in our patients (83.8%). Although a higher proportion of the patients passed away in cohort A (48.6% vs. 17.6%, P=0.030), we did not calculate the estimated OS due to very short median follow-up in cohort B.
The proportion of PD-L1 expression was not significantly different between both cohorts (P=0.47). In cohort B, 4 (36.5%) patients had <1% PD-L1 expression, and ORR was 50% in these patients. Two patients (18.1%) had a PD-L1 expression of >50% in cohort B, and one of them achieved PR whereas another had SD. In patients with PD-L1 expression of >50%, ORR was 80% in the KEYNOTE-021 study and 61.4% in the KEYNOTE-189 study (24,25). Rizvi et al. report ORR of 48% in patients with >1% PD-L1 (measured by BMS’s PD-L1 assay) expression in patients receiving nivolumab and chemotherapy vs. 50% in cohort B of our study with a PD-L1 expression of >1% (28). In patients with PD-L1 expression of >1% receiving pembrolizumab plus chemotherapy, ORR was 53.8% in the KEYNOTE-021 and was 55% in the KEYNOTE-189 study (24,25).ORR was 50% in patient with PD-L1 expression of >1% in cohort B of our study. Since almost similar proportion of the patients responded in our study regardless of PD-L1 expression, these findings suggest that the pembrolizumab have improved outcomes when used in combination with chemotherapy irrespective of the PD-L1 expression.
Brain metastases in NSCLC are not uncommon. Ten percent of the newly diagnosed patients have brain metastases, whereas 30% patients develop brain metastases after initial diagnosis (30). Patients with brain metastasis face therapeutic challenges as the blood-brain barrier penetration of the most chemotherapeutic agents is not well studied. Hence, most of the patients with brain metastases are excluded from the clinical trials as in the KEYNOTE-021 study. Localized therapies (SRS and WBRT) are historically effective in brain metastases (31,32). Goldberg et al. reported the activity of pembrolizumab in untreated NSCLC patients and malignant melanoma. Eighteen untreated or progressed NSCLC patients with brain metastasis showed ORR of 44%. In this trial, the systemic response was 34% (29). In our study, systemic and CNS ORR was higher in cohort B (80% vs. 58.3%, P=0.75 and 80% vs. 41.7%, P=0.14 respectively) despite higher proportion of pre-treated patients in cohort A (83.3% vs. 50% respectively, P=0.13). Further, the time to achieve best response was also shorter in cohort B (1.1 vs. 1.67 months). The sub-group analysis of the objective response by the presence of brain metastasis was not done in the KEYNOTE-189 study.
On excluding the pre-treated patients, 50% patients with brain metastasis in each cohort achieved PR although each cohort had only two untreated patients. Overall, significantly higher proportion of patients with brain metastasis progressed in cohort A [11 (91.7%) vs. 2 (33.3%), P=0.009], but the PFS estimate was not significantly different (P=0.6). Moreover, no patient from cohort B experienced progression of known brain metastases, whereas 4 (36.4%) patients in cohort A experienced progression of known brain metastasis or the emergence of new brain metastatic lesions. One patient from cohort B who had brain metastasis with extensive liver, bone, and bilateral adrenal glands metastases achieved CR. This patient had a PD-L1 expression of 40% and carried KRAS and TP53 mutations. He is 10 months since starting the treatment and continues to do well. In the KEYNOTE-189 study, overall, 75% patients with prior brain metastases progressed and the patients in the pembrolizumab combination did better overall (HR 0.42, 95% CI: 0.26–0.68) (25). In our study, overall 59% patients with known brain metastases progressed and the patients in pembrolizumab combination did better as well.
Although the number of patients with brain metastases in our study is small to reach any meaningful conclusion, we can see a clear a trend towards better outcomes in patients with brain metastases treated with pembrolizumab and chemotherapy. Combination of local therapy with pembrolizumab and the chemotherapy in patients with brain metastases maybe a way moving forward and could be studied in large prospective trials.
Our study has some limitations. The major limitation is a small sample size and convenience sampling. The median follow-up time for the pembrolizumab and chemotherapy combination group is very short. As a result, we did not calculate OS. Due to a small number of patients with significant PD-L1 expression, we could not estimate the effect of PD-L1 expression on the PFS and OS either. Similarly, although we saw a trend toward better outcomes in patients with brain metastases receiving pembrolizumab plus chemotherapy, due to small sample size, the effect was not statistically significant. However, despite limitations, this is a noteworthy analysis of new therapeutic combination in the homogeneous patient population at a large tertiary care cancer center.
Conclusions
Despite the limitations mentioned above, early adoption of this combination of pembrolizumab and dual chemotherapy showed promising results in a real-world setting. The most significant benefit of this combination is observed in the disease progression and stability. Although ORR was similar to that of the KEYNOTE (021 and 189) studies, the difference from chemotherapy only cohort was not significant likely due to a shorter follow-up period of cohort B and small sample size. Although, patients with brain metastases were excluded from the KEYNOTE-021 study, and only asymptomatic or stable previously treated patients with brain metastasis were included in the KEYNOTE-189 study, high ORR, DCR, a lower rate of progression (systemic and local) in patients with brain metastases from cohort B despite having less percentage of pretreated patients suggest a potential benefit of this combination therapy in brain metastases as well. Although, the KEYNOTE-189 study included the patients with brain metastases that were excluded from the KEYNOTE-021 study, treatment-based stratification for response assessment and more detailed analysis in these patients will further elaborate the beneficial effect of this combination of therapies in patients with brain metastases as well. There are several other ongoing phase III studies in NSCLC with brain metastasis that will give definitive answers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by institutional review board (IRB) of Dartmouth-Hitchcock Medical Center/Dartmouth College. Due to retrospective nature of the study, request for informed consent waiver was approved by IRB.
References
- Zukin M, Barrios CH, Pereira JR, et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non–small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 2013;31:2849-53. [Crossref] [PubMed]
- Rossi A, Di Maio M. Platinum-Based chemotherapy in advanced non-Small-Cell lung cancer: optimal number of treatment cycles. Expert Review of Anticancer Therapy 2016;16:653-60. [Crossref] [PubMed]
- Bahl A, Falk S. Meta-analysis of single agents in the chemotherapy of NSCLC: what do we want to know? Br J Cancer 2001;84:1143-5. [Crossref] [PubMed]
- Bunn PA, Kelly K. New chemotherapeutic agents prolong survival and improve quality of life in non-small cell lung cancer: a review of the literature and future directions. Clin Cancer Res 1998;4:1087-100. [PubMed]
- Johnson DH. Evolution of cisplatin-based chemotherapy in non-small cell lung cancer: a historical perspective and the eastern cooperative oncology group experience. Chest 2000;117:133S-137S. [Crossref] [PubMed]
- Splinter TA, Sahmoud T, Festen J, et al. Two schedules of teniposide with or without cisplatin in advanced non-small-cell lung cancer: a randomized study of the European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol 1996;14:127-34. [Crossref] [PubMed]
- McLeod HL, Cassidy J, Powrie RH, et al. Pharmacokinetic and Pharmacodynamic Evaluation of the Glycinamide Ribonucleotide Formyl transferase Inhibitor AG2034. Clin Cancer Res 2000;6:2677-84. [PubMed]
- Prasad DVR, Richards S, Mai XM, et al. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity 2003;18:863-73. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- FDA Approves Dako CDx for Merck's NSCLC Test (Oct. 2015). Retrieved on January 20, 2018. Available online: https://www.genomeweb.com/molecular-diagnostics/fda-approves-dako-cdx-mercks-nsclc-test
- Chatterjee M, Turner DC, Felip E, et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol 2016;27:1291-8. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-Small-Cell lung cancer (POPLAR): a multicentre, open-Label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for non-squamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol 2010;21:1804-9. [Crossref] [PubMed]
- Zitvogel L, Galluzzi L, Smyth MJ, et al. Mechanism of action of conventional and targeted anticancer therapies: reinstating immune-surveillance. Immunity 2013;39:74-88. [Crossref] [PubMed]
- Galluzzi L, Buque A, Kepp O, et al. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015;28:690-714. [Crossref] [PubMed]
- Grabosch S, Zeng F, Zhang L, et al. PD-L1 biology in response to chemotherapy in vitro and in vivo in ovarian cancer. J Immunother Cancer 2015;3:302. [Crossref]
- Zhang P, Ma Y, Lv C, et al. The up-regulation of PD-L1 promotes the resistant response in non-small cell lung cancer patients with neo-adjuvant chemotherapy. Cancer Sci 2016;107:1563-71. [Crossref] [PubMed]
- Gadgeel SM, Stevenson J, Langer CJ, et al. Pembrolizumab (pembro) plus chemotherapy as front-line therapy for advanced NSCLC: KEYNOTE-021 cohorts A-C. Proc Am Soc Clin Oncol 2016;34:Abstr 9016.
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- FDA Approves Pembrolizumab as First-Line Combination Therapy With Pemetrexed and Carboplatin for Metastatic Nonsquamous NSCLC - The ASCO Post, Retrieved on 02/12/2018. Available online: www.ascopost.com/News/55616
- Eisenhauer EA, Therasse P, Bogaerts J, et al. 32 INVITED New response evaluation criteria in solid tumors: revised RECIST guideline version 1.1. EJC Supplements 2008;6:13. [Crossref]
- Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016;34:2969-79. [Crossref] [PubMed]
- Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-Small-Cell lung cancer and untreated brain metastases: early analysis of a non-Randomised, open-Label, phase 2 trial. Lancet Oncol 2016;17:976-83. [Crossref] [PubMed]
- Sørensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol 1988;6:1474-80. [Crossref] [PubMed]
- Mathieu D, Kondziolka D, Cooper PB, et al. Gamma knife radiosurgery for malignant melanoma brain metastases. Clin Neurosurg 2007;54:241-7. [PubMed]
- Redmond AJ, Diluna ML, Hebert R, et al. Gamma Knife surgery for the treatment of melanoma metastases: the effect of intratumoral hemorrhage on survival. J Neurosurg 2008;109:99-105. [PubMed]


