Pre-operative use of aspirin in patients undergoing coronary artery bypass grafting: a systematic review and updated meta-analysis
Introduction
The use of acetylsalicylic acid (aspirin) is associated with a reduction in major adverse cardiovascular events (MACE) and improvement in saphenous vein graft (SVG) patency after coronary artery bypass graft (CABG) surgery (1-5). Despite these benefits, there are still concerns regarding the risk of bleeding when administered in the pre-operative period (1,3,5). The 2012 Society of Thoracic Surgeons guidelines suggest that it may be reasonable to discontinue aspirin for a few (2 to 3) days before CABG to reduce perioperative bleeding and blood transfusions (6). However, there are concerns that discontinuing aspirin in patients who are on chronic therapy prior to surgery may trigger a “rebound phenomenon” in platelet activity that potentially leads to an increased risk of MACE during surgery (7). In this regard, the 2015 American Heart Association Scientific (AHA) Statement recommends that aspirin should be administered pre-operatively and within 6 hours after CABG and be continued indefinitely to reduce SVG occlusion (8).
Randomized controlled trials (RCTs) and previous meta-analyses had been conducted to evaluate the management of aspirin use before CABG (9-15). However, most individual studies were underpowered or yielded conflicting results, which raises concern about the robustness of conclusions. Importantly, the Aspirin and Tranexamic Acid for Coronary Artery Surgery (ATACAS) trial (16) has recently been published providing substantial weight to the current evidence base considering its large sample size. Therefore, an updated systematic review and meta-analysis of RCTs is required to assess clinical outcomes, balance the risks and benefits and thus, enhance decision-making process in this subset of patients. A secondary objective of this review is to explore differences between patients on aspirin with a temporary interruption of the treatment and patients without the interruption in the cohort of patients receiving pre-operative aspirin using an indirect comparison analysis.
Methods
Data sources and searches
A comprehensive literature search on Medline, Embase, PubMed, Cochrane Library and Scopus databases was conducted from conception to November 2016 and a weekly alert for electronic databases was set up until May 9, 2018. The search strategy combined Medical Subject Headings (MeSH) and keywords “aspirin or coronary artery bypass”. The search was not restricted by year of publication or language, and duplicates were removed. When duplicate reports of the same study were identified, only the report with the most complete data and detailed methodology description was included. We also checked reference lists of included RCTs and previous reviews for cross-checking. Table S1 of Supplement provides a list of excluded studies with reasons for exclusion.

Full table
Study selection
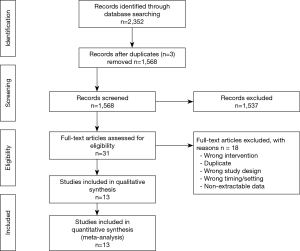
The titles and abstracts yielded by the search were screened independently and in duplicate by two independent investigators (K Solo and T Choudhury) against the inclusion criteria. Any discrepancies between reviewers were resolved by discussion after consulting a third investigator (R Bagur). This systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines (17) (Figure S1).
Eligibility criteria
We included RCTs in which patients undergoing CABG were randomly assigned to pre-operative aspirin or placebo/control before surgery. Patients receiving any dose of aspirin up to the day (within 24 hours) of CABG surgery, regardless of the pre-operative date of initiation and previous duration of aspirin therapy, were considered as the intervention group. Patients receiving placebo or no aspirin before (within 24 hours) CABG surgery, regardless of pre-operative date of initiation and previous duration of aspirin administration, were considered as the control group. Eligible RCTs were required to meet the following criteria: (I) patients undergoing CABG were randomly allocated either to the intervention or the control group; (II) RCTs must not combine aspirin with any other antithrombotic agents in the intervention arm; (III) their primary outcomes must be at least one of the following: mortality, myocardial infarction (MI), chest tube drainage or bleeding, or SVG occlusion; and (IV) extractable data for at least one of these outcomes must be available. When eligible RCTs have more than 2 intervention arms, of which 2 or 3 were eligible, we included all eligible arms.
Data extraction
The full reports of eligible studies were retrieved, and data were extracted independently and in duplicate (K Solo and T Choudhury). Publication details (location, year of publication, author), study and patient characteristics (sample size, length of follow-up, rate of loss to follow-up, aspirin status prior study, demographic and clinical data), procedural characteristics, intervention details (dose, frequency, duration, time of drug administration), and outcomes data were extracted, with differences resolved by discussion with third reviewer (R Bagur).
Quality assessment
Risk of bias was assessed using the Cochrane Risk of Bias tool (18), and the Grading of Recommendation Assessment, Development, and Evaluation (GRADE) system (19) was used to appraise the overall quality of evidence. A summary of quality of evidence was constructed in an evidence profile using GRADEpro software (https://gradepro.org/).
Data synthesis and analyses
Primary outcomes of interest included mortality, peri-operative MI, chest tube drainage and SVG occlusion (per-graft analysis, accounting for clustering effects). Secondary outcomes included need for red blood cell (RBC) transfusions, number of RBC units transfused (one unit of packed-RBC was assumed to be 400 mL), need for surgical re-exploration and stroke.
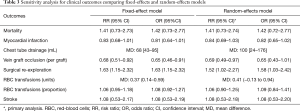
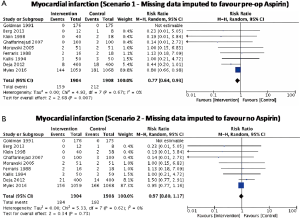
We reported descriptive statistics as percentages for categorical variables and mean [standard deviation (SD)] or median [interquartile range (IQR) or range] for continuous variables. When outcome data were available only as median (IQR or range), mean (SD) were calculated (20,21). Intention-to-treat analysis was followed whenever possible. When a small proportion of studies did not report the uncertainty of point estimates (i.e., SD, IQR, or range), we imputed the missing SDs using single imputation. However, if a large proportion of the data was missing, we set the SD equal to zero (21). Using worst-best sensitivity analysis, we accounted for the missing data of patients who were excluded post randomization and were not analyzed using the original randomized treatment sizes. In best case analysis (scenario 1), we assumed that all excluded patients had the outcome event in the control group, and none in the intervention group, whereas, in worst-case analysis (scenario 2), all excluded patients had the outcome event in the intervention group, and none in the control group. For SVG occlusion endpoint, since grafts within an individual are correlated, we calculated effective sample size (ESS) (which is the new sample size after accounting for clustering effects) instead of the originally reported sample size to account for clustering effects (22). An intra-cluster correlation of 0.177, which was obtained from an external source (23), was used to calculate ESS. Review Manager, version 5.3 (Nordic Cochrane Center, The Cochrane Collaboration) was used to perform pairwise meta-analysis to obtain a pooled estimate of the mean difference (MD) or the risk ratio (RR) and its corresponding 95% confidence interval (CI) with a random-effects model to account for heterogeneity. A continuity correction was used when there were zero events in one of the study arms. When RCTs reported zero events in all study arms for a given endpoint, the correction was not used, but the study was still displayed in the graph for transparency purposes. Post-hoc sensitivity analyses were performed to explore potential differences between random-effects and fixed-effects models and to ascertain the potential influence of studies with high risk of bias on treatment effect.
In addition, we performed an indirect treatment comparison via placebo as a common comparator to explore the impact of prior aspirin use among patients who were randomized to aspirin. Continuously exposed aspirin group was defined as patients receiving aspirin throughout the preoperative period (before and after randomization), whereas interrupted group was defined as patients receiving aspirin who were already on aspirin but had to stop aspirin temporarily due to study protocol until randomization (Figure 1). We performed a meta-analysis involving RCTs of continuously exposed aspirin versus placebo to estimate the treatment effect: RRCP. Another meta-analysis was performed comparing interrupted aspirin versus placebo to obtain RRIP. An indirect estimate was then computed using the following formula and back transformed to obtain the estimated treatment effect of interrupted aspirin versus continuously exposed aspirin: RRIC (24).

where var is the variance of treatment effect.
Post-hoc subgroup analysis was performed to determine whether the dose of aspirin influenced the relative treatment effect. For trials with multiple study arms of different doses, we combined doses of aspirin into a single arm leading to a low-dose (≤100 mg) and high-dose (>100 mg) groups. The Cochrane Q-statistic (I2) was used to assess the consistency among studies, with I2 <25% considered low, I2 25–50% moderate, and I2 >75% high statistical heterogeneity (25). Clinical heterogeneity was also evaluated and described narratively. Both Egger’s test and funnel plot (when >10 studies) were used to examine potential publication bias. Two-sided P values of <0.05 were considered statistically significant.
Results
Study selection, trial and patient characteristics
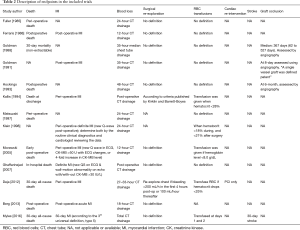
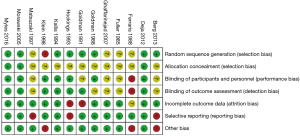
Thirteen RCTs (1,16,26-36) including 4,377 participants met the inclusion criteria (Figure S1). One trial (28) was followed by another publication with longer follow-up information (2). Clinical characteristics and end-points were defined according to study author definitions (Tables 1,2). Overall, 2,266 participants were randomly assigned to pre-operative aspirin (within 24 h of surgery) and 2,111 to control (no aspirin within 24 h of surgery). Age ranged from 53 to 67 years and 85% were male (3,682/4,350) from studies that reported age (1,16,26-36) and sex (1,16,27-36). A total of 996 (29%) patients had diabetes mellitus, 2,937 (71%) hypertension, and 1,194 (32%) previous MI. The vast majority (99%) of participants underwent elective CABG surgery and one trial (27) did not report whether CABG was performed in an elective or urgent setting. On-pump CABG was performed in 95% (3,775/3,984) of patients. Participants received between 2 to 4 grafts per-patient. Three trials (16,33,35) were rated as low-risk of bias, six (1,26,28,30,31,34) as moderate, and four (27,29,32,36) as high (Figure S2).

Full table
Full table
Mortality
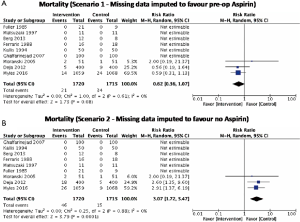
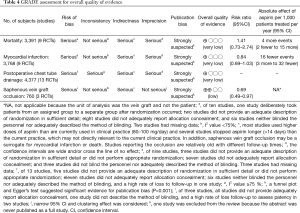
There was no significant difference in effect estimates for 30-day mortality (RR: 1.41, 95% Cl: 0.73–2.74; I2=0%; Figure 2A). Although best case analysis did not alter the findings, worst-case analysis showed a significant increase in the incidence of mortality at 30 days with aspirin (Figure S3A,B). This worst-case scenario is consistent with potential bias due to missing data since the observed point estimate became significant. Sensitivity analysis comparing random-versus fixed-effects suggests no difference in effect estimates between the two models (Table 3). Overall rating of confidence in estimates was very low, due to imprecision, indirectness, risk of bias, missing data, and potential publication bias (Table 4).

Full table
Full table
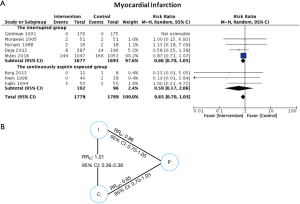
Myocardial infarction
No significant difference in effect estimates was found for peri-operative MI (RR: 0.84, 95% CI: 0.69–1.03, P=0.09; I2=0%; Figure 2B). Although worst case analysis did not alter the findings, best-case analysis suggested a significant reduction in rates of MI (Figure S4A,B). Again, this best-case scenario is consistent with potential bias due to missing data as the conclusion became significant. Sensitivity analysis comparing random- versus fixed-effects suggests no difference in effect estimates between the two models (Table 3). Post-hoc sensitivity analysis confined to trials with low-risk of bias (16,34,36) showed a similar non-significant effect estimates (RR: 0.86, 95% CI: 0.70–1.04; I2=0%). Overall, our confidence in the estimate was very low, owing to indirectness, potential publication bias, and risk of bias due to inadequate randomization, blinding process, missing data, and potential selective reporting of the outcome (Table 4).
Chest tube drainage
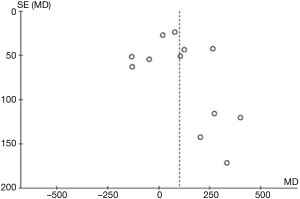
Aspirin was associated with an increased chest tube drainage with a MD of 100.40 mL (95% CI: 24.32–176.47 mL, P=0.01, I2=84%; with imputation for missing standard deviations, Figure 2C). Sensitivity analysis without imputation (MD: 73 mL, 95% CI: −5.04 to 152 mL, P=0.07; I2=80%) significantly altered the effect size. A post-hoc sensitivity analysis suggests no difference between random-versus fixed-effects (Table 3). In summary, our confidence in estimates was very low, due to imprecision, indirectness, and risk of bias due to inadequate randomization, blinding methods, and missing data (Table 4). In addition, the asymmetric funnel plot indicated potential evidence for publication bias (P<0.001; Figure S5).
SVG occlusion
Aspirin was associated with significant treatment effect benefits against SVG occlusion (RR: 0.69, 95% CI: 0.49–0.97, P=0.03, I2=16%) (Figure 2D). Worst- and best-case sensitivity analyses were not performed due to lack of information. Sensitivity analysis comparing random-versus fixed-effects suggests no differences in effect estimates between the two models (Table 3). Overall rating of confidence in estimates was low, owing to indirectness, potential publication bias, and risk of bias due to lack of information about allocation concealment, blinding, different follow-up periods, and high rate of incomplete data (Table 4).
Secondary outcomes
There was an increased risk of surgical re-exploration among patients assigned to pre-operative aspirin (RR: 1.52, 95% CI: 1.02–2.27; P=0.04, I2=8%). No significant differences in effect estimates were found for patients receiving RBC transfusions (RR: 1.06, 95% CI: 0.90–1.25; I2=35%), number of units of RBC transfused (MD: 0.41, 95% CI: −0.13 to 0.94; I2=70%), and stroke (RR: 1.08, 95% CI: 0.53–2.18; I2=0%) (Figure S6).

Indirect comparison
Eight trials reporting MI (16,27,28,30,32,33,35,36) clearly described aspirin exposure prior to the study enrollment. An indirect comparison analysis showed that there was no statistically significant difference in MI between patients who were continuously exposed to aspirin before CABG and those who were not (RR: 1.01, 95% CI: 0.36–2.38) (Figure 3).
Dose of aspirin
Subgroup analysis between low-dose (≤100 mg/day) and high-dose (>100 mg/day) of aspirin showed no significant difference in rates of mortality (interaction P=0.69, I2=0%) and MI (interaction P=0.55, I2=0%). However, a significant statistical interaction of dose was found for chest tube drainage (P=0.05, I2=74.7%) and surgical re-exploration (P=0.04, I2=76.3%) (Figure 4). Overall, the inconsistency of subgroup effect across outcomes reduced our confidence in the credibility of the results.

Discussion
The results of this meta-analysis of 13 RCTs including 4,377 patients undergoing CABG show that pre-operative aspirin reduced the risk of SVG occlusion, but no significant differences in mortality, peri-operative MI and stroke were found. Furthermore, subgroup analysis by dose showed that pre-operative aspirin may produce differential effects on chest tube drainage and surgical re-exploration, but not on mortality nor myocardial infarction. The strategy of continuing aspirin before CABG surgery were also found to be no different from aspirin discontinuation in terms of myocardial infarction. Notably, the strength of the evidence was very low quality, mostly because of high risk of bias; imprecision; indirectness in the applicability of aspirin doses, surrogate outcomes, and timing of discontinuation or restarting; as well as potential for publication bias.
Pre-operative aspirin and outcomes: mortality
Our findings could not confirm or exclude a protective treatment effect of aspirin for mortality and are in line with previous meta-analyses (10,12). This is in contrast to some observational studies which suggested a positive effect on mortality with pre-operative aspirin use (3,5), and a prospective, longitudinal cohort study which suggested aspirin withdrawal before CABG as an independent predictor for mortality (4). The discrepancy may be due to the study design. Nonetheless, the findings of these observation studies and of our analysis remain limited in their interpretation. Even though RCT is considered high-quality evidence, our findings remain inclusive due to low statistical power. Moreover, although the observational data more likely reflect population and settings relevant in real-world clinical practice, they are prone to confounding bias. Well-designed pragmatic trials are therefore warranted to confirm the benefit of pre-operative aspirin on mortality.
Myocardial infarction
Unlike the results of our primary analysis and further sensitivity analysis of trials with low risk of bias, a previous meta-analysis (12) evaluating the effect of aspirin before CABG has suggested significant protective effects for aspirin against MI as compared with control [Peto (odds ratio): 0.79, 95% CI: 0.64–0.99; I2=0%]. However, this meta-analysis included trials that assessed aspirin in combination with other antiplatelet therapy and trials that were not designed to measure clinical outcomes. Inclusion of such studies may have introduced significant variability in study protocols across trials and augment the treatment effect on MI, allowing for statistical significance to be reached. Moreover, the rationale of using Peto OR to estimate the relationship between interventions and outcome is unclear. According to a simulation study, Peto OR is appropriate to use when event rates are below 1% (37). However, the incidence rate of MI reported in that meta-analysis was between 9% and 11%. It is notable that the upper limit of the CI in the previous meta-analysis for MI was borderline significant, and therefore should be interpreted cautiously as the estimate may not be adequately robust to provide definitive conclusions for clinical practice.
However, our finding is in agreement with recent meta-analyses (14,15) that showed no significant benefit of pre-operative aspirin against MI, though the evidence is of very low quality. In the absence of high quality evidence of protective effect, clinicians should now consider other aspects (e.g., patient’s value and preference, perceived risk, resources) when providing an optimal prophylactic management plan to the patients.
Chest tube drainage
Previous observational studies and meta-analysis (3,5,12) showed that the use of pre-operative aspirin was associated with an increased risk of blood loss, surgical re-exploration and RBC transfusions. These findings are consistent with our analysis, with the exception that the likelihood of RBC transfusions did not reach statistical significance in our study. Notably, the observed increased risk of blood loss was mostly found in trials allocating participants to higher doses of aspirin (>100 mg/day). However, the clinical significance of an increased risk of 100 mL blood loss is certainly questionable.
SVG patency
It is well-established that SVG occlusion has been the limiting factor of long-term outcome after CABG. Although the risk of occlusion increases with time, early occlusion occurs more commonly. Thus, providing early prophylaxis against the occlusion, especially, during pre-operative period conceptually has promising results. Our meta-analysis shows that pre-operative aspirin provides a significant benefit to SVG patency, which is a plausible finding given the anticipated antiplatelet effect of aspirin.
However, the available data is insufficient to recommend an optimal dose of aspirin, although a possible larger protective effect with a medium-dose (300–325 mg/day) in reducing graft occlusion compared to low-dose (50–150 mg/day) of aspirin was documented in an indirect comparison meta-analysis (38). Nonetheless, due to the observational nature of the study design, these data should be interpreted with caution.
Aspirin with or without temporary interruption before CABG in the intervention group
There was a variation in study protocols between studies in terms of stopping aspirin use prior to study enrollment among patients receiving pre-operative aspirin. Our indirect comparison analysis failed to show a significant difference in MI between aspirin with temporary interruption and without the interruption before CABG among patients receiving pre-operative aspirin. In contrast, EACTS guidelines (39) have recently recommended the continuation of aspirin throughout the pre-operative period in patients on aspirin who are undergoing CABG to reduce ischemic events. However, the recommendation was based on class IIa and level of evidence C. Certainly, the evidence is not robust enough to make a definite conclusion. Nonetheless, further research is warranted to confirm the benefit and risk of continuing aspirin in CABG patients on aspirin.
Clinical implication
Observational studies (40-42) investigating on the association of SVG occlusion and clinical outcomes have suggested that there may be an association between SVG occlusion and clinical outcomes. However, the current evidence fails to provide a sufficient connection between SVG patency and clinical outcomes. Despite a significant reduction in occlusion, these data fail to support the hypothesis that pre-operative aspirin protects patients undergoing CABG from mortality and MI. In the absence of high quality evidence of protective effect of pre-operative aspirin on patient-relevant outcomes and continuation of aspirin in patient on aspirin, clinicians should now consider patient’s value and preference when providing an optimal prophylactic management plan to the patients.
Strengths and limitations
The strengths of our work include a comprehensive literature search, restriction to RCTs, duplicate evaluation of eligibility and data abstraction, risk of bias tool, and the use of GRADE system for quality of evidence assessment.
The present study has several limitations. The results of this analysis should be interpreted considering its limitations. The main limitation lies with the small number of studies, patients and events informing each outcome of interest. Most studies were short-term trials with duration up to 30 days post-CABG; therefore, the treatment effects beyond one month remain uncertain for relevant patient-important clinical outcomes and SVG patency. Moreover, the incidence of SVG occlusion was measured at very different time-points (8 to 527 days). Other limitations include the high loss-to-follow-up rate, which may overestimate the results due to the potential risk of selection bias, and the relatively old studies reporting the occlusion data, which may not reflect the recent clinical practice. Hence, the clinical interpretation of our finding remains limited by the potential bias due to missing data. Furthermore, our indirect comparison analysis may be underpowered to show a significant impact of wash-out period prior to randomization among patients receiving pre-operative aspirin. Additionally, our indirect treatment comparison is observational by nature, therefore, the result of our analysis may be at risk of confounding bias. Well-designed head-to-head comparative studies may therefore be needed to provide a definitive answer to the question of whether we should continue or stop aspirin before CABG. Moreover, since trials included in this meta-analysis primarily consisted of elective CABG patients, it limits our ability to generalize our findings to higher-risk patients such as individuals admitted with acute coronary syndrome and undergoing CABG during the index hospitalization, where the risk for ischemic events is significantly higher. Lastly, patient-level data were not available, precluding therefore, a more robust adjustment for any differences in clinical and surgical/procedural variables (i.e., on-pump versus off-pump or use of antifibrinolytics), or a combined clinical end point (i.e., composite outcome) that used for statistical convenience.
Conclusions
Our results suggest that pre-operative aspirin before CABG surgery is associated with lower risk of SVG occlusion, though no significant differences in clinical outcomes were found. Furthermore, aspirin dose may induce differential effects on chest tube drainage and surgical re-exploration; however, the effects of dose and aspirin interruption on mortality and MI are still unclear. These data are based on trials where the strength of evidence consists of low to very-low quality, therefore, well-designed RCTs are needed to provide a more reliable estimate for patient-important outcomes.
Acknowledgements
A special thanks to Dr. Per O. Vandvik, Department of Medicine, Innlandet Hospital Trust, Gjøvik, Norway, for his constructive comments that greatly contributed to improving the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Goldman S, Copeland J, Moritz T, et al. Improvement in early saphenous vein graft patency after coronary artery bypass surgery with antiplatelet therapy: results of a Veterans Administration Cooperative Study. Circulation 1988;77:1324-32. [Crossref] [PubMed]
- Goldman S, Copeland J, Moritz T, et al. Saphenous vein graft patency 1 year after coronary artery bypass surgery and effects of antiplatelet therapy. Results of a Veterans Administration Cooperative Study. Circulation 1989;80:1190-7. [Crossref] [PubMed]
- Dacey LJ, Munoz JJ, Johnson ER, et al. Effect of preoperative aspirin use on mortality in coronary artery bypass grafting patients. Ann Thorac Surg 2000;70:1986-90. [Crossref]
- Mangano DT. Multicenter Study of Perioperative Ischemia Research Group. Aspirin and mortality from coronary bypass surgery. N Engl J Med 2002;347:1309-17. [Crossref]
- Bybee KA, Powell BD, Valeti U, et al. Preoperative aspirin therapy is associated with improved postoperative outcomes in patients undergoing coronary artery bypass grafting. Circulation 2005;112:I286-92. [PubMed]
- Ferraris VA, Saha SP, Oestreich JH, et al. 2012 update to the Society of Thoracic Surgeons guideline on use of antiplatelet drugs in patients having cardiac and noncardiac operations. Ann Thorac Surg 2012;94:1761-81. [Crossref] [PubMed]
- Lordkipanidze M, Diodati JG, Pharand C. Possibility of a rebound phenomenon following antiplatelet therapy withdrawal: a look at the clinical and pharmacological evidence. Pharmacol Ther 2009;123:178-86. [Crossref] [PubMed]
- Kulik A, Ruel M, Jneid H, et al. Secondary prevention after coronary artery bypass graft surgery: a scientific statement from the American Heart Association. Circulation 2015;131:927-64. [Crossref] [PubMed]
- Alghamdi AA, Moussa F, Fremes SE. Does the use of preoperative aspirin increase the risk of bleeding in patients undergoing coronary artery bypass grafting surgery? Systematic review and meta-analysis. J Card Surg 2007;22:247-56. [Crossref] [PubMed]
- Sun JC, Whitlock R, Cheng J, et al. The effect of pre-operative aspirin on bleeding, transfusion, myocardial infarction, and mortality in coronary artery bypass surgery: a systematic review of randomized and observational studies. Eur Heart J 2008;29:1057-71. [Crossref] [PubMed]
- Ma X, Ma C, Yun Y, et al. Safety and efficacy outcomes of preoperative aspirin in patients undergoing coronary artery bypass grafting: a systematic review and meta-analysis. J Cardiovasc Pharmacol Ther 2014;19:97-113. [Crossref] [PubMed]
- Hastings S, Myles P, McIlroy D. Aspirin and coronary artery surgery: a systematic review and meta-analysis. Br J Anaesth 2015;115:376-85. [Crossref] [PubMed]
- Hastings S, Myles P, McIlroy D. Aspirin and coronary artery surgery: an updated meta-analysis. Br J Anaesth 2016;116:716-7. [Crossref] [PubMed]
- Sa M, Soares AF, Miranda RGA, et al. Stopping versus continuing acetylsalicylic acid before coronary artery bypass surgery: A systematic review and meta-analysis of 14 randomized controlled trials with 4499 patients. Eur J Cardiothorac Surg 2017;52:838-47. [Crossref] [PubMed]
- Aboul-Hassan SS, Stankowski T, Marczak J, et al. The use of preoperative aspirin in cardiac surgery: A systematic review and meta-analysis. J Card Surg 2017;32:758-74. [Crossref] [PubMed]
- Myles PS, Smith JA, Forbes A, et al. Stopping vs. Continuing Aspirin before Coronary Artery Surgery. N Engl J Med 2016;374:728-37. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9, W64.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Higgins J, Green S. editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. Available online: http://handbook.cochrane.org. Accessed 19 December 2016.
- Killip S, Mahfoud Z, Pearce K. What is an intracluster correlation coefficient? Crucial concepts for primary care researchers. Ann Fam Med 2004;2:204-8. [Crossref] [PubMed]
- Nazarian SM. Predictor of early saphenous vein graft patency, platelet hyper-reactivity and aspirin-insensitive thromboxane generation in patients undergoing coronary artery bypass graft surgery. Johns Hopkins University, 2009.
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Fuller JK, Copeland JG. Does short-term pre-operative aspirin in coronary bypass patients increase post-operative bleeding? Vasc Endovascular Surg 1985;19:174-8.
- Ferraris VA, Ferraris SP, Lough FC, et al. Preoperative aspirin ingestion increases operative blood loss after coronary artery bypass grafting. Ann Thorac Surg 1988;45:71-4. [Crossref] [PubMed]
- Goldman S, Copeland J, Moritz T, et al. Starting aspirin therapy after operation. Effects on early graft patency. Circulation 1991;84:520-6. [Crossref] [PubMed]
- Hockings BE, Ireland MA, Gotch-Martin KF, et al. Placebo-controlled trial of enteric coated aspirin in coronary bypass graft patients. Effect on graft patency. Med J Aust 1993;159:376-8. [PubMed]
- Kallis P, Tooze JA, Talbot S, et al. Pre-operative aspirin decreases platelet aggregation and increases post-operative blood loss--a prospective, randomised, placebo controlled, double-blind clinical trial in 100 patients with chronic stable angina. Eur J Cardiothorac Surg 1994;8:404-9. [Crossref] [PubMed]
- Matsuzaki K, Okabe H, Kajihara N, et al. A prospective study of the timing of discontinuation of aspirin before coronary artery bypass grafting. Nihon Kyobu Geka Gakkai Zasshi 1997;45:1710-4. [PubMed]
- Klein M, Keith P, Dauben H, et al. Aprotinin counterbalances an increased risk of peri-operative hemorrhage in CABG patients pre-treated with Aspirin. Eur J Cardiothorac Surg 1998;14:360-6. [Crossref] [PubMed]
- Morawski W, Sanak M, Cisowski M, et al. Prediction of the excessive perioperative bleeding in patients undergoing coronary artery bypass grafting: role of aspirin and platelet glycoprotein IIIa polymorphism. J Thorac Cardiovasc Surg 2005;130:791-6. [Crossref] [PubMed]
- Ghaffarinejad MH, Fazelifar AF, Shirvani SM, et al. The effect of preoperative aspirin use on postoperative bleeding and perioperative myocardial infarction in patients undergoing coronary artery bypass surgery. Cardiol J 2007;14:453-7. [PubMed]
- Deja MA, Kargul T, Domaradzki W, et al. Effects of preoperative aspirin in coronary artery bypass grafting: a double-blind, placebo-controlled, randomized trial. J Thorac Cardiovasc Surg 2012;144:204-9. [Crossref] [PubMed]
- Berg K, Langaas M, Ericsson M, et al. Acetylsalicylic acid treatment until surgery reduces oxidative stress and inflammation in patients undergoing coronary artery bypass grafting. Eur J Cardiothorac Surg 2013;43:1154-63. [Crossref] [PubMed]
- Bradburn MJ, Deeks JJ, Berlin JA, et al. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 2007;26:53-77. [Crossref] [PubMed]
- Lim E, Ali Z, Ali A, et al. Indirect comparison meta-analysis of aspirin therapy after coronary surgery. BMJ 2003;327:1309. [Crossref] [PubMed]
- Sousa-Uva M, Head SJ, Milojevic M, et al. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg 2018;53:5-33. [Crossref] [PubMed]
- Alexander JH, Hafley G, Harrington RA, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA 2005;294:2446-54. [Crossref] [PubMed]
- Lopes RD, Mehta RH, Hafley GE, et al. Relationship between vein graft failure and subsequent clinical outcomes after coronary artery bypass surgery. Circulation 2012;125:749-56. [Crossref] [PubMed]
- Halabi AR, Alexander JH, Shaw LK, et al. Relation of early saphenous vein graft failure to outcomes following coronary artery bypass surgery. Am J Cardiol 2005;96:1254-9. [Crossref] [PubMed]


