Thermic and chemical procedures for bronchoscopic lung volume reduction
Introduction
The chronic obstructive pulmonary disease (COPD) is a disease spread world-wide with a high morbidity and mortality. An estimated 65 million patients suffer from a moderate to severe COPD, with approximately 5 million deaths in 2005 (1). Due to the chronic inflammatory process, emphysematous destruction of the lung parenchyma and bronchoconstriction, the airflow is limited and can cause a hyperinflation, accompanied with symptoms like cough, dyspnoea, low exercise capacity and a reduced quality of life. There is no curative therapy available at present, thus the recent therapeutic options focus on slowing down the progression of the disease and relieving the symptoms. Basic components are anti-obstructive pharmacotherapy, physical exercise, oxygen therapy and non-invasive ventilation.
In the 1950s, Brantigan et al. developed the lung volume surgery (LRVS) to improve the respiratory mechanics by reducing hyperinflation leading to better exercise capacity und lung function. Due to a rather high morbidity and mortality, this procedure was abandoned until the 1990s and reintroduced by Cooper et al. The most comprehensive trail regarding the LVRS is the National Emphysema Treatment Trail (NETT), which compared 608 patients who underwent a LVRS vs. 610 patients treated with a conservative medical therapy (2). Surgically treated patients displayed improvements in pulmonary function, exercise capacity a quality of life.
In the past 14 years several endoscopic approaches could be developed with comparable physiological effects like LVRS but less attendant risk. One of these procedures is endoscopic valve therapy, which is mostly used in Europe and Australia and imitates LVRS effects by placing one-way valves in the most emphysematous lobe to reach a complete atelectasis. The efficacy could be demonstrated in various randomized controlled trials (RCT) (3-7). A disadvantage of the valve therapy is the need of complete interlobar fissures and thus the absence of collateral ventilation (CV). Another technique for endoscopic lung volume reduction is the treatment with coils. In contrast to valve therapy, this therapeutic approach is independent of CV. The efficacy regarding improvements in lung function, exercise capacity and quality of life could be proved in various RCTs (8-10). However, in the biggest RCT, known as “RENEW”, coil therapy compared with usual care resulted in a median improvement in exercise tolerance that was modest and of uncertain clinical importance (10).
In addition to the mentioned ELVR techniques, there are two further procedures, which can be effectively used independent of interlobar CV in patients with upper lobe predominant emphysema. These techniques are described in detail below.
Bronchoscopic thermal vapor ablation (BTVA)
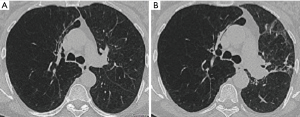
The BTVA (Uptake Medical Corporation, Seattle, WA, USA) was first described in 2009 and belongs to the non-blocking, irreversible techniques. It is applied to patients with predominant upper lobe emphysema by instilling heated water vapor at a temperature of 75 °C in the preselected lung areas (Figure 1). It induces an inflammatory reaction with a following fibrosis and shrinkage of the treated lobe/segment with resulting lung volume reduction (Figure 2). As against valve or coil technique, the BTVA can be used on segmental level with the intention to treat the most diseased lung segments and to protect the healthier ones. Another advantage is the independence of CV (11).
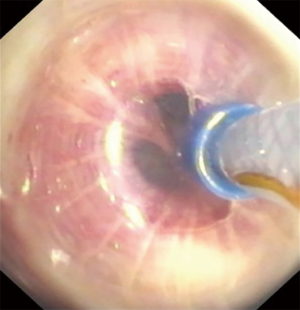
Before treatment with BTVA, a careful patient selection is necessary with following criteria:
- heterogeneous, upper-lobe predominant emphysema;
- symptomatic despite adequate medical therapy;
- FEV1 (forced expiratory volume in 1 second) 20–45%, RV (residual volume) >150%, DLco (diffusion capacity) >20% and 6-minute walk test (6-MWT) >140 metres.
To assess the severity and distribution of the emphysema, a multi-detector computed tomography (MDCT) has to be performed. The targeted segment—the most emphysematous segments—has to be detected by its density and volume, based on the MDCT using specialised software (InterVapor Personalized Procedure Program, IP3) for processing the data to a 3D reconstruction of the patient’s airways. Thus, the needed dose of heated vapor and the treatment time is calculated, too.
For the procedure, the InterVapor catheter is inserted in a conventional flexible bronchoscope and placed in the airway of the targeted lung segment. Before instilling the vapor, a balloon at the distal tip of catheter is inflated to occlude the proximal airways. Afterwards, the vapor, generated by the InterVapor generator, is instilled within 3–10 seconds, depending on the calculated vapor amount.
The BTVA system itself contains two parts, including the InterVapor generator, an electronically controlled vessel pressure to generate the vapor and to deliver the calculated amount, and the InterVapor catheter, to occlude the proximal airways and lead the vapor into the targeted segment. Between the treatments of two areas, an interval of 3 minutes should be kept.
The first BTVA prospective single arm trial in 2009 confirmed the feasibility and safety in patients with predominant upper-lobe emphysema (12). In 2012, a more comprehensive single arm trial, 44 emphysema patients were treated unilaterally on lobar level (13). It showed a significant improvement of FEV1 (141±26 mL), RV (−406 ±113 mL) and quality of life [St. George’s Respiratory Questionaire (SGRQ) 14±2.4 points). In this single-arm trial, it could be shown, that the BTVA-induced local inflammatory reaction (LIR) can be accompanied by symptoms like cough, fever, sputum, dyspnoea and haemoptysis (12,13), associated with increasing inflammatory markers (C-reactive-protein, white blood-cell body count and neutrophils) and local X-ray changes. Of 44 patients treated with BTVA, 16 had adverse respiratory events during the first 30 days with the need of pharmaceutical therapy (antibiotics/glucocorticosteroids). The peak of a LIR was reached 2–4 weeks after lobar BTVA. However, a review analysis demonstrated that especially in these patients, a greater lobar volume reduction (65.3% vs. 33.4%; P=0.007) with a lower RV (−933 vs. 13 mL; P<0.001) could be measured in a 12 months follow-up (14). Accordingly, FEV1 (166 vs. 48 mL), exercise capacity (38.5 vs. 9.3 m) and quality of life (SGRQ −12.2 vs. −10.5 pts.) improved in the 12 months interval.
The most recent and first RCT was published in 2016, known as “STEP-UP” trial investigating the end point FEV1 and SGRQ (15,16). Seventy patients with predominant upper-lobe emphysema were enrolled and randomly assigned: 45 in the treatment group (n=5 unilateral; n=40 bilateral) and 24 in the control group. This time the BTVA was performed on segment level instead of on lobar level, with the intention to protect the less severely damaged segments and to minimize the LIR. The treated segments had a density (tissue-to-air-ratio) of 8.5% (SD, 1.8%), while untreated, healthier segments had a density of 10.4% (SD, 2.2%). In a 6-month follow-up, the mean between group difference of FEV1 was 14.7% (P<0.0001) with an absolute difference of 130.8 mL (63.6–198.0; P=0.0002). The mean difference in SGRQ was −9.7 points (P=0.0021) and in RV reduction −302.5 mL (−542.6 to 62.4; P=0.0145). Thus, it could be revealed that a targeted therapy on segment level of the more destroyed areas is superior to a medical treatment with a significant improvement regarding lung function and quality of life. However, in a 180-day control period, a total of 28 treated patients received serious adverse events leading to hospital admission. Most common events were COPD exacerbation and pneumonia/pneumonitis. In one patient a pneumothorax occurred, but without the need of surgical treatment. One patient died caused by a COPD exacerbation 84 days after treatment. This death was reviewed and was judged to be possibly related to treatment. All other occurred events could be managed with standard therapy; intensive care treatment was not needed.
Summarizing, BTVA presents independent of CV an alternative effective minimally invasive treatment for patients with predominant upper-lobe emphysema. The special advantage is the possibility to treat on segment level, preserving healthier parts of the lobe.
Polymeric lung volume reduction
The PLVR is an alternative non-blocking, irreversible approach for patients with advanced predominant upper lobe emphysema, which was first used in 2011. The technique is similar to BTVA in terms of the intention to induce an inflammatory reaction, following by a fibrosis and shrinkage of the treated area leading to volume reduction.
Prior to the intervention, the emphysema heterogeneity and targeted treatment sides have to be identified by MDCT. During the procedure with a conventional flexible bronchoscope in wedge position on sub segment-level, the emphysematous lung synthetic polymer sealant (ELS; 4.5 mL polymer substrate, 0.5 mL cross-linker, foamed to a total of 20 mL) is delivered via catheter. After injection, the position is maintained for 1 minute to accomplish complete polymerization (Figure 3). Afterwards, the bronchoscope is positioned to next treatment side.

The first single-arm, non-controlled trails, published in 2011 and 2012, revealed improvements of lung function and quality of life, despite just a small number of treated patients (17,18).
The first and so far the only RCT is known as “ASIPRE” and was published in 2015 (19). In this trial, 57 patients (n=34 treated, n=23 control) with advanced upper-lobe predominant emphysema were enrolled and received an ELS in two upper-lobe sub-segments after CT-based screening. Due to the aforementioned experiences from the pilot studies, all treated patients received a 7-day pre-interventional glucocorticosteroid therapy and a per-interventional antibiotic prophylaxis to reduce the risk of LIR. The primary endpoint of efficacy was intended to be a change of FEV1. Secondary end-points were exercise capacity (6-MWT), quality of life (SGRQ), dyspnoea (mMRC) and upper lobe volume. An improvement of FEV1 by 11.4% after 3 months and 18.9% after 6 months in the treated group vs. −2.1% or 1.3% in the control group could be revealed. Significant improvements could be measured in the 6-MWT (31.0 vs. −22.0 m) and SGRQ (−12 vs. −3 pts.) at 6 months follow-up as well. However, there was no difference in the dyspnoea score. Despite a pre- and per-interventional medical prophylaxis, 44% of treated patients experienced adverse events with the need of hospital admission and pharmacotherapy. The most frequent symptoms were pneumonia, COPD-exacerbations, respiratory failure and LIRs. Two patients died; one 55 days after intervention caused by myocardial infarction, another one 65 days post-procedure due to pneumonia. Also in this trial, a tendency to a correlation between adverse respiratory events and FEV1 improvement (responders 67% events vs. 27% non-responders) was shown.
Due to economic reasons, the trial had to be prematurely cancelled and this technique has not been available since 2013. Currently, PLVR has been resumed in the course of a new multicenter study known as “STAGE” (NCT02877459). Data is not yet available and is expected to be published in 2018.
PLVR may present a further possible therapeutic option for patients with predominant upper-lobe emphysema. The advantage is the independence of CV. However, fewer data is available and the risk profile is unfavourable. Further research seems necessary regarding the amount of the instilled polymer and its composition. The predictive factors have to be re-evaluated as well to optimize patient selection and improve benefit-risk-profile.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. 2017.
- Fishman A, Martinez F, Naunheim K, et al. A randomized trail comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [Crossref] [PubMed]
- Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233-44. [Crossref] [PubMed]
- Herth FJ, Noppen M, Valipor A, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J 2012;39:1334-42. [Crossref] [PubMed]
- Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomized controlled trial. Lancet 2015;386:1066-73. [Crossref] [PubMed]
- Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015;373:2325-35. [Crossref] [PubMed]
- Valipour A, Slebos DJ, Herth F, et al. Endobronchial valve therapy in patients with homogeneous emphysema. Results from the IMPACT Study. Am J Respir Crit Care Med 2016;194:1073-82. [Crossref] [PubMed]
- Shah PL, Zoumot Z, Singh S, et al. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respir Med 2013;1:233-40. [Crossref] [PubMed]
- Deslee G, Klooster K, Hetzel M, et al. Lung volume reduction coil treatment for patients with severe emphysema: a European multicenter trial. Thorax 2014;69:980-6. [Crossref] [PubMed]
- Sciurba FC, Criner GJ, Strange C, et al. Effect of endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema: the RENEWRandomized Clinical Trial. JAMA 2016;315:2178-89. [Crossref] [PubMed]
- Gompelmann D, Eberhardt R, Schuhmann M, et al. Lung volume reduction with vapor ablation in the presence of incomplete fissures: a 12-month results from the STEP-UP Randomized Controlled Study. Respiration 2016;92:397-403. [Crossref] [PubMed]
- Snell GI, Hopkins P, Westall G, et al. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg 2009;88:1993-8. [Crossref] [PubMed]
- Snell G, Herth FJ, Hopkins P, et al. Bronchoscopic thermal vapor ablation therapy in the management of heterogeneous emphysema. Eur Respir J 2012;39:1326-33. [Crossref] [PubMed]
- Gompelmann D, Eberhardt R, Ernst A, et al. The localized inflammatory response to bronchoscopic thermal vapor ablation. Respiration 2013;86:324-31. [Crossref] [PubMed]
- Herth FJ, Valipour A, Shah PL, et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6-month results of the multicenter, parallel-group, open-label, randomized controlled STEPUP trial. Lancet Respir Med 2016;4:185-93. [Crossref] [PubMed]
- Herth FJ, Ernst A, Baker KM, et al. Characterization of outcomes one year after endoscopic thermal vapor ablation for patients with heterogeneous emphysema. Int J Chron Obstruct Pulmon Dis 2012;7:397-405. [PubMed]
- Herth FJ, Gompelmann D, Stanzel F, et al. Treatment of advanced emphysema with emphysematous lung sealant (AeriSeal®). Respiration 2011;82:36-45. [Crossref] [PubMed]
- Kramer MR, Refaely Y, Maimon N, et al. Bilateral endoscopic sealant lung volume reduction therapy for advanced emphysema. Chest 2012;142:1111-7. [Crossref] [PubMed]
- Come CE, Kramer MR, Dransfield MT, et al. A randomized trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J 2015;46:651-62. [Crossref] [PubMed]


