Is alectinib the new first line therapy in ALK-rearranged advanced non-small cell lung cancer?
Since the registration of crizotinib for ALK-positive NSCLC in 2010, the landscape of testing and drug development has changed enormously (1). Fluorescence in situ hybridization (FISH) has been used as golden standard for a long time. However, recently ALK-IHC (immunohistochemistry) with D5F3 antibody showed to be a better predictor of tumor response to crizotinib than FISH (2). Therefore, D5F3 has been used in most recently conducted clinical trials and is one of the most favorable tests to screen for ALK in non-squamous NSCLC at this moment.
Until recently crizotinib was the only first-line therapy for ALK-positive NSCLC. Other drugs, e.g., alectinib and ceritinib, could be used in subsequent treatment lines when resistance occurred due to crizotinib (3,4). Additional ALK inhibiting drugs, e.g., brigatinib, lorlatinib, TPX-0005, and X-396, are studied in phase 1–3 trials, and we are awaiting results of those trials (Table 1) (5-8).
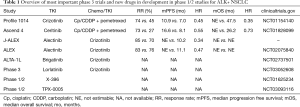
Full table
Last year results from J-ALEX and ALEX trials have been presented and published. Because these trials have impact on the current treatment landscape, we here discuss both trials.
J-ALEX trial
Hida et al. showed in a phase 3 trial that alectinib was favorable compared to crizotinib in a solely Japanese population with ALK rearranged advanced NSCLC in first and second line (9). Screening was conducted with IHC, FISH and RT-PCR. In 5 patients all 3 tests were positive, in 215 patients IHC and FISH were positive and 14 patients had a positive RT-PCR alone. In total 207 patients were randomized to alectinib (N=103) and crizotinib (N=104). Age, sex, disease stage and line of treatment were well balanced between both groups. Sixty-four percent of patients received treatment in first line and 36% received 2nd line treatment. More (asymptomatic) brain metastases were found in the crizotinib group compared to the alectinib group (28% vs. 14%). Median progression free survival (mPFS), which was the primary endpoint, was not yet reached for the group treated with alectinib (95% CI, 20.3– not estimable; NE) compared to 10.2 months (95% CI, 8.2–12.0) for the group treated with crizotinib, with a hazard ratio (HR) of 0.34 (99.7% CI, 0.17–0.71). The group treated in first line showed a mPFS for alectinib of more than 17.5 months (95% CI, 17.5– NE) compared to crizotinib of 10.2 months (95% CI, 8.3–13.9), leading to HR 0.31 (95% CI, 0.17–0.57). The group treated in second line showed a mPFS for alectinib of 20.3 months (95% CI, 20.3– NE) compared to crizotinib of 8.2 months (95% CI, 6.4–15.7), leading to HR 0.40 (95% CI, 0.19–0.87). Overall survival data are immature at this moment. Subgroup analysis showed that patient with brain metastases without prior whole brain radiotherapy (WBRT) had an impressive better PFS while on alectinib compared to crizotinib [HR 0.10 (95% CI, 0.01–0.77)]. There was no statistical difference for mPFS comparing both drugs in patients who had WBRT before starting ALK inhibitors [HR 0.38 (95% CI, 0.04–3.35)]. However, this group was rather small (16 vs. 6 patients) so no definitive conclusion can be given. In the alectinib-treated patients less gastrointestinal disorders, e.g., decrease appetite, nausea, vomiting and diarrhea, and eye disorders have been observed compared to crizotinib-treated patients. Also less abnormal laboratory tests were observed for ASAT, ALAT, neutrophil count, and QTc prolongation. Blood bilirubin increase and myalgia were more seen in alectinib treated patients.
ALEX trial
Peters et al. showed in a phase 3 trial with a mixed population of Asian and non-Asian patients that alectinib (n=152) had a superior efficacy compared to crizotinib (n=151) in first line treatment (10). The primary endpoint of the study was PFS. Only patients with a positive D5F3 IHC were included. Randomization was stratified for ECOG PS, race and presence of brain metastases at baseline. Brain imaging was obligatory, however CT of the brain was permitted, where MRI is the golden standard for excluding small brain metastases. In the alectinib- and the crizotinib-treated group brain metastases were present in 58 patients and 64 patients, respectively. Treatment with radiotherapy was performed in 22 and 27 patients, respectively. Other baseline characteristics were balanced as well.
The investigator assessed that median (m) PFS for alectinib-treated patients was at least 17.7 months (95% CI, 17.7– NE) compared to 11.1 months (95% CI, 9.1–13.1) for crizotinib-treated patients, HR 0.47 (95% CI, 0.34–0.65; P<0.001). The independent review committee assessed mPFS was 25.7 months (95% CI, 19.9– NE) and 10.4 months (95% CI, 7.7–14.6), respectively with a HR of 0.50 (95% CI, 0.36–0.70; P<0.001).
Patients with prior brain metastases responded better to alectinib compared to crizotinib: 17/21 (81%; 95% CI, 58–95) vs. 11/22 (50%; 95% CI, 28–72). HR for PFS in patients with brain metastases was 0.40 (95% CI, 0.25–0.64).
OS data are immature at this moment. Safety data are comparable to J-ALEX data and therefore alectinib appears to have less toxicity compared to crizotinib.
Discussion
Both J-ALEX and ALEX trials met their primary endpoints. Alectinib has a much favorable PFS compared to crizotinib with an improved efficacy on non-radiated brain metastasis. In both studies no systematic use of MRI was performed to detect brain metastases, and no difference was shown between alectinib treated groups with and without observed brain metastases. That limits the sensitivity of detecting brain metastasis, however, the mPFS for alectinib was favorable compared to the crizotinib-treated group. Since brain metastases are invalidating, life threatening and heavily decrease the quality of life, alectinib is the preferred option for first-line ALK positive advanced NSCLC. This can be the same for other drugs that are capable to pass the blood-brain barrier, e.g., brigatinib and lorlatinib, but we have to await final study results to draw conclusions on those drugs. Crizotinib has a low intracranial response of about 26%. P-glycoprotein is an ABCB1 transporter located at the blood-brain that is a barrier for crizotinib, ceritinib and brigatinib, but not alectinib. Intracranial tumor response to alectinib is 81%. The estimated total mPFS of sequential crizotinib followed by alectinib at progressive disease is 20 months. This is even less than the mPFS of alectinib in first line treatment (25.7 months). Moreover, not all patients that progressed on crizotinib will reach treatment with alectinib. These arguments are in favor of first-line alectinib followed at disease progression by an approach with biopsies to determine the resistance mechanism for subsequent treatments. Whether overall survival should be the primary endpoint for estimating sequential schedules of ALK inhibitors is questionable from a patient’s perception given better quality of life, less brain metastases, and less toxicity.
Toxicity is less for patients treated with alectinib compared to crizotinib, especially for gastrointestinal side effects. Ethnic differences in alectinib pharmacokinetics were noted. In the J-ALEX trial, the Japanese patients received a lower dose (300 mg BID instead of 600 mg BID for Western countries) due to the four times lower AUC0-10 in the US patients than in Japanese patients with ALK positive NSCLC. The underlying differences are not fully solved (3,11). Since daily side effects can have great impact on quality of life, alectinib has many favorable characteristics compared to crizotinib.
In conclusion, alectinib should become the new first line standard treatment for ALK-positive NSCLC. Whether there is still a role for crizotinib in the treatment of ALK-positive NSCLC is not clear, it has a role for MET amplifications or mutations associated with exon 14 skipping and ROS1rearrangements at this moment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. Erratum in: N Engl J Med 2011;364:588. [Crossref] [PubMed]
- van der Wekken AJ, Pelgrim R, 't Hart N, et al. Dichotomous ALK-IHC Is a Better Predictor for ALK Inhibition Outcome than Traditional ALK-FISH in Advanced Non-Small Cell Lung Cancer. Clin Cancer Res 2017;23:4251-8. [Crossref] [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [Crossref] [PubMed]
- Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 2014;4:662-73. [Crossref] [PubMed]
- Cui JJ, Zhai D, Deng W, et al. TPX-0005, a novel ALK/ROS1/TRK inhibitor, effectively inhibited a broad spectrum of mutations including solvent front ALK G1202R, ROS1 G2032R and TRKA G595R mutants. Eur J Cancer 2016;69:S32. [Crossref]
- Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med 2016;374:54-61. [Crossref] [PubMed]
- Pall G. The next-generation ALK inhibitors. Curr Opin Oncol 2015;27:118-24. [Crossref] [PubMed]
- Zhang S, Anjum R, Squillace R, et al. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin Cancer Res 2016;22:5527-38. [Crossref] [PubMed]
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Gadgeel SM, Shaw AT, Govindan R, et al. Pooled Analysis of CNS Response to Alectinib in Two Studies of Pretreated Patients With ALK-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:4079-85. [Crossref] [PubMed]


