Management of incidental lung nodules <8 mm in diameter
Introduction
Pulmonary nodules are common. Since introduction of helical computed tomography (CT) in the early 1990s and multidetector row CT in the late 1990s, the detection of nodules as small as 1–2 mm in diameter has become routine. In fact, the majority of smokers who undergo thin- section CT have been found to have small lung nodules, most of which are smaller than 7 mm in diameter (1). In the National Lung Screening Trial 39% of participants had a positive finding defined as a non-calcified pulmonary nodule larger than 4 mm (2). The clinical importance of these extremely small nodules differs substantially from that of larger nodules detected on chest radiographs. These small nodules in the vast majority are benign.
Due to the increase of incidentally detected pulmonary nodules and the information obtained from several screening programs, updated guidelines and recommendations for the management of small pulmonary nodules have been proposed. Pulmonary nodule management guidelines are based on size, density and patient risk. These updated guidelines coincide in proposing periodic follow up for small nodules, less than 8 mm of diameter (3,4). On the other hand, the new 8th edition of the tumor, node and metastasis (TNM) classification for lung cancer establishes that each centimeter is important and separates cancers smaller than 1 cm as T1a (5). In this paper we review the management of pulmonary nodules focused in the incidentally detected pulmonary nodules smaller than 8 mm.
Pulmonary nodules characteristics
A pulmonary nodule is a rounded or irregular opacity, which may be well or poorly defined, measuring ≤3 cm in diameter (6). A pulmonary nodule is considered small if its largest diameter is 10 mm or less. A micronodule is considered a pulmonary nodule <3 mm (6,7). Most nodules smaller than 1 cm are not visible on chest radiographs and are only visible by CT. Nodules are classified into three main categories based on their density, solid and subsolid nodules; subsolid nodules (SSNs) include non-solid nodules and part solid nodules (PSN). Solid nodules are seen most frequently, and they are of soft tissue density and obscure the contour of vessels with which they are in contact. Non-solid nodules or pure ground glass nodules (GGNs) are focal nodular areas of increased lung attenuation, including both well and poorly defined lesions, which do not obscure normal parenchymal structures, including airways and vessels. PSN or mixed nodules are a combination of both ground-glass and solid components, the latter obscuring underlying lung architecture (6).
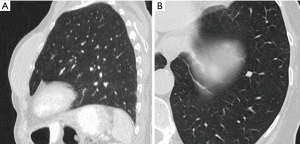
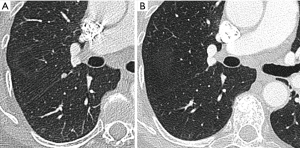
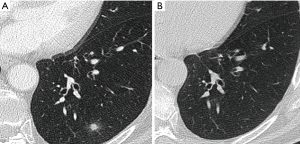
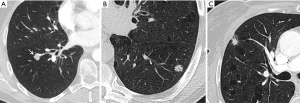
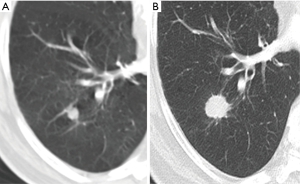
Most small solid pulmonary nodules are benign, 80% granulomas and intrapulmonary lymph nodes, 10% hamartomas and 10% other benign lesions. Calcification in a small nodule favors a benign cause, central, laminar, pop corn or diffuse patterns of calcification are reliable evidence of benignancy (Figure 1) (8). Calcifications may be observed rarely in lung cancer, they use to be amorphous or punctate (9) and pulmonary metastases from a primary bone-forming malignancy can be densely calcified. Fat content suggests a hamartoma or occasionally a lipoid granuloma or lipoma (Figure 1) (10). Intrapulmonary lymph nodes are very common pulmonary nodules, they use to be located below the carina, they have oval, round, triangular, or trapezoidal shape with sharply defines borders; solid and homogenous density and frequently had a discrete thin tag extending to the pleura (11). They frequently are located perifissural. Typical perifissural lymph nodes are defined as a fissure-attached, homogeneous, solid nodule with smooth margins and an oval, lentiform, or triangular shape. Perifissural nodules are benign (12,13). Multiplanar reformations (MPR) are very useful in the evaluation of perifissural nodules and intrapulmonary lymph nodes (Figure 2). Consider follow-up of larger intrapulmonary lymph nodes, especially in the presence of a known extrapulmonary malignancy (4), and in perifissural nodules that do not meet all typical features e.g. spiculated borders or nodules with a shape that does not appear to be influenced by the fissure (12) (Figure 3).
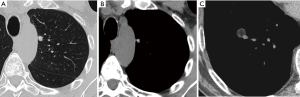
Solid pulmonary nodules without these benign characteristics (calcium, fat or perifissural) are indeterminate. Well-defined, smooth and regular margins suggest nodule benignancy (14). However, 21% of malignant nodules have well-defined and regular margins (8) (Figure 4). Ill-defined, irregular or especially spiculated margins suggest malignancy. It is important to know that malignant and benign nodules associated with emphysema exhibited considerably more overlap in CT features than did nodules in non-emphysematous lungs (15).
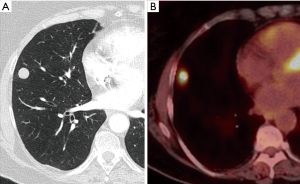
SSNs have different implications from solid nodules. Transient SSNs are frequent and of inflammatory origin (Figure 5). In a recent study 27% of suspicious SSNs were transient (16). Persistent subsolid pulmonary, including both GGNs and PSNs, are mostly malignant lesions (17). They potentially manifest as early adenocarcinomas; the size of the ground glass component correlates with the lepidic component and the solid component has an important correlation with the histologic characteristics of invasive adenocarcinomas (18). This radiopathologic correlation has more relevance since the 2011 IASLC/ATS/ERS classification of lung adenocarcinoma (Table 1) (19,20), later adopted by the 2015 WHO Classification (21). The radiologic characterization of the subsolid pulmonary nodules is also applied in the Eighth Edition of the TNM Classification of Lung Cancer (22) with the recommendation that the T category according to tumor size should be determined measuring the size of the solid (invasive) component (22). At the present time, all guidelines have different and specific management protocols for subsolid nodules.

Full table
Measuring pulmonary nodules
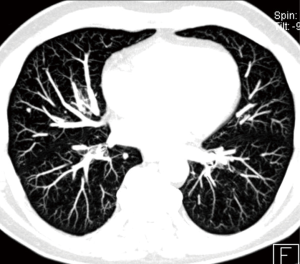
To evaluate pulmonary nodules, and especially small pulmonary nodules less than 8 mm in diameter, it is necessary to perform a chest CT with thin sections ≤1.5 mm reconstructed with MPR and maximum intensity projection (MIP) with both soft tissue and pulmonary filter. MIP reformations are useful in the detection of small pulmonary nodules (Figure 6), increasing the sensitivity and reducing the number of overlooked small nodules, particularly in the central lung (23,24).
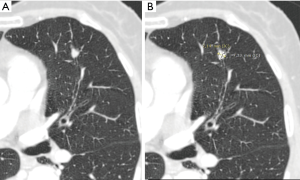
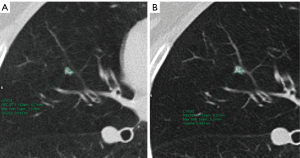
The likelihood of malignancy in a pulmonary nodule correlates strongly with both its size and its growth rate, allowing for additional factors, such as a history of prior lung cancer or extrathoracic malignancy (25). Size is a very important predictor of malignancy in pulmonary nodules, so it has to be measured as accurately as possible. To measure pulmonary nodules, the largest diameter, the mean diameter or the volume can be used. The Fleischner Society states that mean diameter is better for risk estimation (7). Mean diameter is the average of long and perpendicular short axis diameters in the same place, measured in the axial plane (Figure 7). Mean diameter is best for risk estimation and correlate better with tumor volume than one measurement, particularly in elongated nodules and in nodules where the short dimension is better defined (26). For measuring small pulmonary nodules and SSNs, it is better to use lung window setting with a high spatial frequency (sharp) filter (17). Measurements should be expressed to the nearest mm, for example, a nodule with a mean diameter of 7.6 mm should be expressed as a 8 mm nodule (7). Measuring with electronic calipers has an inter and intrareader variability; when observers measured nodules 20 mm in diameter or smaller, the limits of inter- and intrareader variability were 1.73 and 1.32 mm, respectively (27). Given this variability measuring diameters, the Fleischner Society recommends to report growth when a change in diameter of at least 2 mm is detected (7). It is possible to do volumetric nodule measurements. Their potential advantages are: (I) volume measurements may better encapsulate the three-dimensional nature of a pulmonary nodule; (II) volume estimation allows for calculation of the volume doubling time (VDT), a parameter that is proposed to more reliably define nodule growth; and (III) it reduces the inconsistency between and among observers measuring diameters (28). To perform a good volumetric assessment is important to maintain consistency of acquisition and reconstruction (especially section thickness and reconstruction algorithm) and it is desirable to perform sequential nodule evaluations with the identical software type and version (7,28). Subsolid nodules and nodules attached to pleura or vessels are especially difficult to evaluate by means of volumetry due to their difficult segmentation (28). The addition of nodule volume to existing malignancy prediction models increases the proportion of nodules correctly classified (29). All the current guidelines are including volumetric assessment in their recommendations. The British Thoracic Society (BTS) added initial volume and volume doubling time (VDT) calculations to the diameter, and the Fleischner Society added volume to diameter in its latest updated guidelines (3,4). To confirm volume growth in a nodule it has to be superior to 25%, due to the fact that volume changes ≤25% may be due to interscan variability (30,31). Growth detection is better and early depicted by volumetric assessment compared to diameter. Small changes in diameter can represent important changes in volume (Figure 8); for spherical masses, a 25% increase in diameter corresponds to a doubling of overall volume (32).
Follow-up CT has to be performed with low-dose technique of no more than 3 mGy. In follow-CT the aim is to evaluate nodule persistence and eventual growth rate.
Pulmonary nodules and risk of cancer
There are different parameters to assess the probability of malignancy in pulmonary nodules, including radiological and clinical predictors. The first radiological predictor of malignancy is size. The average risk of cancer in solid nodules smaller than 6 mm (100 mm3) in patients at high risk is less than 1%, and for nodules measuring 6–8 mm (250 mm3) there is an estimated average risk of malignancy of approximately 0.5–2.0% (33). The cancer risk is much lower in low-risk patients. The cancer risk increases in pulmonary nodules larger than 8 mm. In the NELSON study the risk of malignancy in nodules larger than 10mm was 15.2% (34). Other radiological nodule features are spiculation, pleural indentation, nodule growth (Volume doubling time <400 days.) and upper lobe location (4). In small nodules is difficult to assess specific radiological features; as nodules become larger, their morphology becomes more distinct, and management should be strongly influenced by the appearance of the nodule rather than by size alone (Figure 9) (3). In the evaluation of SSNs, to include radiological criteria as internal structure, presence of bullae, solid core characteristics, borders or surrounding tissue increases substantially the malignancy rate compared with the average risk based on nodule type and size (16). The NELSON study showed that new solid nodules (incident not prevalent) have a high probability of malignancy even at a small size and should be followed up more aggressively than known nodules or nodules detected at baseline (35).
Growth rate is an important predictor of malignancy (Figure 10). Growth rate is better estimated by volumetry and VDT. Software-calculated pulmonary nodule VDT of more than 500 days has a 98% negative predictive value for the diagnosis of solid malignant pulmonary nodules; and VDTs in the range of 20–400 days have been reported for malignant solid nodules (Figure 8) (36). In the NELSON screening trial, growing nodules were stratified in risk groups according to VDT (high risk <400 days; intermediate risk 400–600 days; low risk >600 days) (37). SSNs represent slow growing cancer and manifest longer VDT.
Age and smoking are the classical clinical risk factors for lung cancer. Age is clearly related with the risk of cancer. Lung cancer is uncommon in individuals younger than 35 years and is unusual before the age of 40 years. The likelihood of malignancy increases more than twofold for every 10-year increase in age (38). Cigarette smoking has been established as the major risk factor for lung cancer since the 1960s; current or former smokers were approximately eight times more likely than never-smokers to have malignant nodules (38). A smoking history of 30 pack-years or more and quitting smoking within the past 15 years have been used as the qualifying tobacco exposure threshold for the NLST screening program, and they should be considered indicative of high-risk status in patients with solid nodules (2). Other risk factors are emphysema, idiopathic pulmonary fibrosis and known extrapulmonary or pulmonary malignancy. All these risk factors are more useful and applicable in solid pulmonary nodules than in SSNs.
Some of these features have been included into prediction models to characterize pulmonary nodules. Currently the American College of Chest Physicians (ACCP) guidelines suggest using the Mayo Clinic prediction model based on patient categorization into low (>5%), intermediate (5–65%) and high risk (>65%) of malignancy (25), while the BTS guidelines (4) suggest the use of the Brock (33) and Herder models (39).
Nodules <8 mm and the guidelines
Guidelines for management of pulmonary nodules on CT are general recommendations, not orders, and have to be applied in each patient individually.
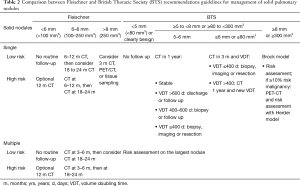
Fleischner and BTS guidelines are the most recent and popular guidelines for incidental pulmonary nodules management (3,4). Guidelines have specific recommendations according to nodule characteristics (density and size) and cancer risk of the patient. Regarding density, both guidelines separate recommendations for solid and subsolid nodules (Tables 2,3).
Full table

Full table
The recently updated Fleischner Society guidelines are the most extended worldwide (Tables 2,3). These guidelines manage the nodules based on density, size, number of nodules (solitary or multiple) and the patient’s cancer risk. It is important that patients younger than 35 years or with known extrapulmonary malignancy are excluded from the guidelines, being not applicable in oncologic staging or in a lung cancer screening program.
In solid nodules smaller than 8 mm, the recommendation is to do CT follow-up and in nodules larger than 8 mm the recommendation is consider performing CT in 3 months, PET-CT or tissue sampling. The establishment of this cut-off in 8 mm is based on the risk of malignancy. Time surveillance is dependent on the initial nodule size and the patient risk. The larger the diameter of the nodule, the greater the patient's risk and the shorter the time interval of follow-up. For pulmonary nodules smaller than 8 mm, the follow-up protocol will depend on the risk of the patient (high or low risk) and if the size is below 6 mm or between 6 and 8 mm. Nodules less than 6 mm in low risk patient do not require follow-up. Depending on the patient’s risk, the CT follow-up can be performed from 3 to 24 months. Multiple small nodules are also managed based on patient’s risk and nodule size. In this scenario of multiple nodules the follow-up is closer to rule out pulmonary metastases (Figure 11).
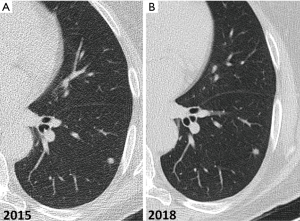
Non-calcified solid nodules are followed for a period of two years because 2-year stability implies benignity (40) (Figure 12). In SSNs a longer follow-up period is required because many of them are indolent or slow growing neoplasms (Figure 13). GGNs with diameter ≥6 mm should be followed-up for 5 years, with time scan intervals of 2 years, while PSN with a solid component <6 mm should be evaluated annually for 5 years. PSNs with a solid component ≥6 mm after an initial follow-up are highly suspicious of invasive malignancy.
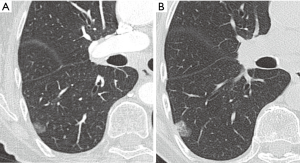
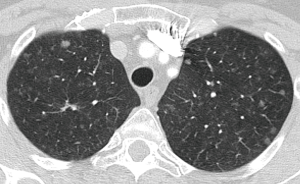
BTS guidelines are based on size by means of nodule volumetry and VDT, and cancer risk calculated by Brock Model (33) or by Herder Model including PET-CT findings (39). These guidelines cover adults (≥18 years) with pulmonary nodules and one of the major difference from Fleischner guidelines is that they do not exclude nodules in patients with current or previously treated malignancy, or nodules detected in a CT screening for lung cancer. This is based on the fact that no studies were found that compared the features of pulmonary nodules according to the route of presentation. They exclude from surveillance nodules with benign appearance and perifissural and subpleural nodules.
BTS guidelines (Tables 2,3) use the cut-off of 5 mm or 80 mm3 for solid and subsolid nodules, giving the fact that in the NLST and NELSON trials, the prevalence of lung cancer among patients with 4–6 mm nodules was 0.5%. Nodules less than 5 mm do not require follow-up. In the case of solid nodules, the recommendation is to offer CT surveillance to people with nodules ≥5 to <8 mm maximum diameter or ≥80 to <300 mm3 and use a prediction model, the Brock model, for initial risk assessment of pulmonary nodules ≥8 mm or ≥300 mm3. If the risk of malignancy is ≥10%, a PET-CT with risk assessment using Herder model is recommended. As seen in Table 1, the follow-up of a 5–6 mm pulmonary nodule is annually and in the case of a 6–8 mm nodule, the follow-up is earlier compared to Fleischner guidelines, being at 3 months, giving the fact that at this time CT will reliably detect growth in larger nodules, and will also demonstrate resolution in the majority of resolving nodules. After this, the follow-up mainly depends on the VDT. Automated or semi-automated volumetry is more accurate than diameter measurements and accuracy of VDT assessment is better after 1 year than 3 months, especially for small nodules (<6 mm). In the context of multiples pulmonary nodules the recommendations is to base the risk assessment on that of the largest nodule (Figure 11). In the CT follow-up VDT has to be calculated to decide management: according to VDT there are different options; (I) VDT >600 days, consider discharge or CT follow-up; (II) 400–600 days, consider biopsy or CT follow-up; and (III) VDT ≤400 days, consider resection or local treatment.
Also in the case of subsolid nodules, recommendations differ from the Fleischner guidelines, being the follow-up earlier, at 3 months, with a thin section CT, and if the nodule is stable, it assesses the risk of malignancy (Brock model) to decide the follow-up.
Other pulmonary nodules management guidelines come from the American College of Chest Physicians (25) and specific guidelines for lung cancer screening as Lung-Rads from The American College of Radiology (ACR) (41) and Lu-Rads (42) from Canadian Authors.
None of these guidelines is specifically addressed to nodules in patients with a history of an oncologic disease. Most of guidelines exclude oncologic patients, making the management of nodules in this patient group particularly challenging. In a recent survey sent to all members of the Society of Thoracic Radiology regarding criteria for the management of incidentally detected lung nodules in oncologic patients, radiologists tend to report every detected nodule and to routinely recommend follow-up CT examinations (43). In this setting 75.84% of the respondents recommend short term follow-up CT for every incidentally detected nodule, being nodule size the most important factor determining follow-up intervals (43). In oncologic patients the usual recommendation is a 3 to 6 months follow-up due to the shortest VDT of metastases compared with primary tumors. In oncologic patients, the same as in non-oncologic patients, baseline nodules (prevalent) have a lower malignancy risk than new or incident (not incidental) nodules (Figures 14,15) (35,44). Prevalent nodules are common in oncologic patients and many of them are not malignant. The incidence of indeterminate pulmonary nodules on staging chest CT in patients with colorectal cancer varied from 4% to 42%, and the majority (70%) did not have any clinical significance (45). There are some radiological findings that increase the probability of malignancy of prevalent nodules in patients with cancer: nodules larger than 6–7 mm, number of nodules, contour irregularity and presence of pleural studding (44,46).
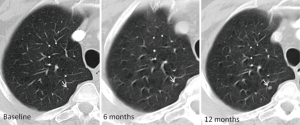
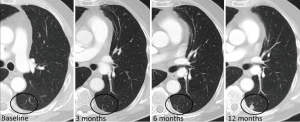
Biopsy of small pulmonary nodules
Percutaneous biopsy is a very useful technique in the management of pulmonary nodules (48). Cytological or histological specimens may be obtained with fine needle aspiration biopsy (FNAB) or with core needle biopsy (CNB) devices, respectively. Biopsy procedures are more difficult in small nodules (<10 mm) than in larger nodules (Figure 16). Ng et al. demonstrated that CT-guided FNAB is a useful tool in the diagnosis and management of small pulmonary nodules (49). In this paper, they reported sensitivity for malignancy and overall accuracy of 67.7% and 78.8%, respectively. Pneumothorax occurred in 52.7% of the patients, with 9.1% patients requiring a pleural drainage. Despite the lower diagnostic accuracy and higher complication rate than those of larger pulmonary lesions, CT-guided FNAB should be considered as a useful tool in the management of small nodules that require histological confirmation before treatment. One of the challenges while performing a percutaneous lung biopsy is the displacement of the nodule due to the respiratory movement. Tomiyama et al. demonstrated that a respiratory gating technique increases the diagnostic accuracy of percutaneous CT biopsy in nodules <15 mm from 69% (without gating) to 96% (with gating) (50). Wallace et al. demonstrated that lesions between 0.8 and 1.0 cm that are not subpleural offer the best opportunity for success (51).
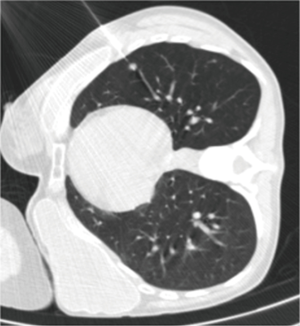
Choo et al. obtained good results in the diagnosis of small pulmonary nodules (<1 cm) using C-arm cone-beam CT guidance (52). The sensitivity, specificity, and diagnostic accuracy of cone-beam CT percutaneous biopsy were 96.7%, 100% and 98%, respectively, with only a 6.5% rate of pneumothorax, and 5.6% of hemoptysis. Using C-arm cone-beam CT, Hwang et al. did not find significant differences in sensitivity, specificity and accuracy between percutaneous biopsy of nodules with a size <1 cm or larger nodules (from 1 to 2 cm) (53).
Preoperative localization of small lung nodules
There are no guidelines concerning which nodules should be resected using preoperative localization. Ciriaco et al. recommend localizing nodules smaller than 10 mm and located more than 15 mm of the pleura (54). The study performed by Nakashima et al. suggested that preoperative localization should be considered before video-assisted thoracoscopic surgery operation, if the pulmonary nodule meets two or more of these criteria: (I) maximum diameter of the nodule of 5 mm or less; (II) maximum diameter to minimum distance between the visceral pleural and inferior border of nodule of 0.5 or less; and (III) nodule with low-density (55).
There is no consensus about the method to localize pulmonary nodules. Hook wire is a safe and effective procedure that can be performed in a one-day surgery program (Figure 17) (56). In the meta-analysis done by Park et al. comparing localization with hook wire, microcoil, and lipiodol, the authors found that all three methods yielded similarly highly successful targeting rates (96%, 97%, and 99%, respectively) (57). Hook wire had a relatively lower successful operative field targeting rate because of dislodgement or migration; lipiodol showed the highest overall success rate; and microcoil localization yielded the lowest complication rate (57).
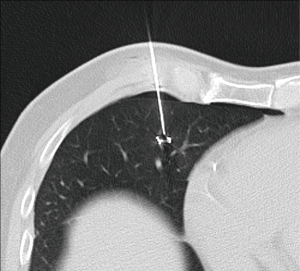
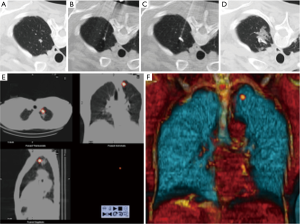
Zaman et al. compared the different techniques of preoperative localization and found that finger palpation showed suboptimal results and should be avoided (57). They concluded that radio-guided surgery is a preferable method (Figure 18). It showed high accuracy with minimal complications and operator dependence in detecting subcentimeter pulmonary nodules when compared with other techniques such as ultrasonography, finger palpation, fluoroscopic, hook wire, spiral wire and microcoil localization (58).
Conclusions
Small pulmonary nodules are common and most of them are benign. Guideline recommendations for these nodules agree to perform follow-up CT, except for low risk patients with nodules smaller than 5–6 mm. Nodules larger than 8 mm require an active approach. The intermediate group of nodules between 6–8 mm or in high risk patients, follow-up is mandatory to assess stability or growth. Subsolid nodules have specifics recommendations in guidelines due to its association with the spectrum of lung adenocarcinoma. Regarding measuring pulmonary nodules, volumetry and VDT seem better than caliper diameter to assess growth in small nodules. The caveat of volumetry is that it is necessary to maintain acquisition characteristics and the same software evaluation to obtain good results.
In some specific circumstances, as high-risk patient, nodule characteristics or oncologic patient, follow-up is not the preferred option and an interventional approach to these nodules is performed. The monitoring of pulmonary nodules in cancer patients remains a topic to be defined.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Swensen SJ. The probability of malignancy in solitary pulmonary nodules. Arch Intern Med 1997;157:849. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Callister MEJ, Baldwin DR, Akram AR, et al. BTS guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70 Suppl 2:ii1-54. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Bankier AA, MacMahon H, Goo JM, et al. Recommendations for Measuring Pulmonary Nodules at CT: A Statement from the Fleischner Society. Radiology 2017;285:584-600. [Crossref] [PubMed]
- Erasmus JJ, Connolly JE, McAdams HP, et al. Solitary pulmonary nodules: Part I. Morphologic evaluation for differentiation of benign and malignant lesions. Radiographics 2000;20:43-58. [Crossref] [PubMed]
- Grewal RG, Austin JH. CT demonstration of calcification in carcinoma of the lung. J Comput Assist Tomogr 1994;18:867-71. [Crossref] [PubMed]
- Gaerte SC, Meyer CA, Winer-Muram HT, et al. Fat-containing lesions of the chest. Radiographics 2002;22:S61-78. [Crossref] [PubMed]
- Shaham D, Vazquez M, Bogot NR, et al. CT features of intrapulmonary lymph nodes confirmed by cytology. Clin Imaging 2010;34:185-90. [Crossref] [PubMed]
- de Hoop B, van Ginneken B, Gietema H, et al. Pulmonary perifissural nodules on CT scans: rapid growth is not a predictor of malignancy. Radiology 2012;265:611-6. [Crossref] [PubMed]
- Ahn MI, Gleeson TG, Chan IH, et al. Perifissural Nodules Seen at CT Screening for Lung Cancer. Radiology 2010;254:949-56. [Crossref] [PubMed]
- Ost D, Fein A. Evaluation and management of the solitary pulmonary nodule. Am J Respir Crit Care Med 2000;162:782-7. [Crossref] [PubMed]
- Matsuoka S, Kurihara Y, Yagihashi K, et al. Peripheral solitary pulmonary nodule: CT findings in patients with pulmonary emphysema. Radiology 2005;235:266-73. [Crossref] [PubMed]
- Chung K, Jacobs C, Scholten ET, et al. Lung-RADS Category 4X: does it improve prediction of malignancy in subsolid nodules? Radiology 2017;284:264-71. [Crossref] [PubMed]
- Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer. AJR Am J Roentgenol 2002;178:1053-7. [Crossref] [PubMed]
- Lee KH, Goo JM, Park SJ, et al. Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J Thorac Oncol 2014;9:74-82. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC lung cancer staging project: Proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2016;11:1204-23.
- Gruden JF, Ouanounou S, Tigges S, et al. Incremental benefit of maximum- intensity-projection images on observer detection of small. AJR Am J Roentgenol 2002;179:149-57. [Crossref] [PubMed]
- Kawel N, Seifert B, Luetolf M, et al. Effect of slab thickness on the CT detection of pulmonary nodules: Use of sliding thin-slab maximum intensity projection and volume rendering. AJR Am J Roentgenol 2009;192:1324-9. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-120S.
- Nair A, Baldwin DR, Field JK, et al. Measurement methods and algorithms for the management of solid nodules. J Thorac Imaging 2012;27:230-9. [Crossref] [PubMed]
- Revel M-P, Bissery A, Bienvenu M, et al. Are two-dimensional CT measurements of small noncalcified pulmonary nodules reliable? Radiology 2004;231:453-8. [Crossref] [PubMed]
- Devaraj A, van Ginneken B, Nair A, et al. Use of volumetry for lung nodule management: theory and practice. Radiology 2017;284:630-44. [Crossref] [PubMed]
- Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest 2014;145:464-72. [Crossref] [PubMed]
- Field JK, Duffy SW, Baldwin DR, et al. UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161-70. [Crossref] [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT Scanning. N Engl J Med 2009;361:2221-9. [Crossref] [PubMed]
- Field JK, Oudkerk M, Pedersen JH, et al. Prospects for population screening and diagnosis of lung cancer. Lancet 2013;382:732-41. [Crossref] [PubMed]
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369:910-9. [Crossref] [PubMed]
- Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014;15:1332-41. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, de Jong PA, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: Analysis of data from the randomised, controlled NELSON trial. Lancet Oncol 2016;17:907-16. [Crossref] [PubMed]
- Revel MP, Merlin A, Peyrard S, et al. Software volumetric evaluation of doubling times for differentiating benign versus malignant pulmonary nodules. AJR Am J Roentgenol 2006;187:135-42. [Crossref] [PubMed]
- Xu DM, Gietema H, de Koning H, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006;54:177-84. [Crossref] [PubMed]
- Gould MK, Ananth L, Barnett PG. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest 2007;131:383-8. [Crossref] [PubMed]
- Herder GJ, van Tinteren H, Golding RP, et al. Clinical prediction model to characterize pulmonary nodules. Chest 2005;128:2490-6. [Crossref] [PubMed]
- Slattery MM, Foley C, Kenny D, et al. Long-term follow-up of non-calcified pulmonary nodules (<10 mm) identified during low-dose CT screening for lung cancer. Eur Radiol 2012;22:1923-8. [Crossref] [PubMed]
- ACR. LungRADS Assessment Criteria. 2014;1. Available online: http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/LungRADS/AssessmentCategories.pdf
- Manos D, Seely JM, Taylor J, et al. The Lung Reporting and Data System (LU-RADS): a proposal for computed tomography screening. Can Assoc Radiol J 2014;65:121-34. [Crossref] [PubMed]
- Occhipinti M, Heidinger BH, Pfannenberg C, et al. Managing incidental lung nodules in patients with a history of oncologic disease. J Thorac Imaging 2017;32:115-20. [Crossref] [PubMed]
- Hammer MM, Mortani Barbosa EJ. Predictive factors for malignancy in incidental pulmonary nodules detected in breast cancer patients at baseline CT. Eur Radiol. Eur Radiol 2017;27:2802-9. [Crossref] [PubMed]
- Parnaby CN, Bailey W, Balasingam A, et al. Pulmonary staging in colorectal cancer: A review. Colorectal Dis 2012;14:660-70. [Crossref] [PubMed]
- Pomerri F, Pucciarelli S, Maretto I, et al. Significance of pulmonary nodules in patients with colorectal cancer. Eur Radiol 2012;22:1680-6. [Crossref] [PubMed]
- O JH, Yoo IeR, Kim SH, et al. Clinical Significance of Small Pulmonary Nodules with Little or No 18 F-FDG Uptake on PET / CT Images of Patients with Nonthoracic Malignancies. J Nucl Med 2007;48:15-21. [PubMed]
- Vollmer Torrubiano I, Sánchez González M . Interventional procedures in the chest. Radiologia 2016;58 Suppl 2:15-28. [Crossref] [PubMed]
- Ng YL, Patsios D, Roberts H, et al. CT-guided percutaenous fine-needle aspiration biopsy of pulmonary nodules measuring 10 mm or less. Clin Radiol 2008;63:272-7. [Crossref] [PubMed]
- Tomiyama N, Mihara N, Maeda M, et al. CT-guided neele biopsy of small pulmonary nodulaes: value of respiratory gating. Radiology 2000;217:907-10. [Crossref] [PubMed]
- Wallace MJ, Krishnamurthy S, Broemeling LD, et al. CT-guided percutaneous fine-needle aspiration biopsy of small (< or =1-cm) pulmonary lesions. Radiology 2002;225:823-8. [Crossref] [PubMed]
- Choo JY, Park CM, Lee NK, et al. Percutaneous transthoracic needle biopsy of small (<1 cm) lung nodules under C-arm cone-beam CT virtual navigation guidance. Eur Radiol 2013;23:712-9. [Crossref] [PubMed]
- Hwang HS, Chung MJ, Lee JW, et al. C-arm cone-beam CT-guided percutaneous transthoracic lung biopsy: usefulness in evaluation of small pulmonary nodules. AJR Am J Roentgenol 2010;195. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodulaes: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Nakashima S, Watanabe A, Obama T, et al. Need for preoperative computed tomography-guided localization in video-assisted thoracoscopic surgery pulmonary resections of metastatic pulmonary nodules. Ann Thorac Surg 2010;89:212-8. [Crossref] [PubMed]
- Molins L, Mauri E, Sánchez M, et al. Locating pulmonary nodules with a computed axial tomography-guided harpoon prior to videothoracoscopic resection. Experience with 52 cases. Cir Esp 2013;91:184-8. [Crossref] [PubMed]
- Park CH, Han K, Hur J, et al. Comparative effectiveness and safety of preoperative lung localization for pulmonary nodules: A systematic review and meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]
- Zaman M, Bilal H, Woo CY, et al. In patients undergoing video-assisted thoracoscopic surgery excision, what is the best way to locate a subcentimetre solitary pulmonary nodule in order to achieve successful excision? Interact Cardiovasc Thorac Surg 2012;15:266-72. [Crossref] [PubMed]


