Uniportal video-assisted thoracoscopy major lung resections after neoadjuvant chemotherapy
Introduction
Neoadjuvant therapy in the treatment of advanced stages non-small-cell lung cancer (NSCLC) is well established and part of the guidelines (1-3). This enables a better survival rate (4,5). However, surgery after the application of induction therapy is associated with higher complication rates compared to surgery alone (6). Several complications are also due to the surgical approach itself. The optimal surgical approach for these cases is still under investigation. Due to just few studies, the evidence for a long-term survival for patients with video assisted thoracoscopy (VATS) vs. patients with an open approach after chemotherapy is still outstanding (7,8).
The uniportal VATS is now well developed in several experienced thoracic centers worldwide. This technique is used not only for minor but also for major resections in thoracic surgery. Several case reports describing advanced complicated cases operated in a uniportal VATS technique were published recently in the literature (9). Few non-randomized studies showed that uniportal VATS can be superior to multiportal VATS and conventional surgery in terms of postoperative complications (10). However, the role of uniportal VATS for cases after neoadjuvant therapy is still scientifically not well-defined (11). The already described benefits of uniportal VATS like direct vision and reach of the target tissue as well as less complication rates could play a significant role in cases after induction therapy (7,12).
This single center study elucidates the preliminary short-term results for uniportal VATS in terms of safety of the technique, survival and complications, investigating any possible risk factor influencing outcomes.
Methods
Between June 2012 and September 2017, 642 Uniportal VATS procedures were performed at Thoracic Surgery Department, Charité-Universitätsmedizin Berlin (Germany). Among these, 154 patients underwent a uniportal VATS anatomical lung resection for primary lung cancer: 136 without a preoperative neoadjuvant treatment and 18 after induction chemotherapy. The prospectively collected clinical data of all these patients were retrospectively reviewed and analyzed.
The clinical evaluation of the patients before the neoadjuvant therapy included: routine blood tests, electrocardiography, radiological and diagnostic examinations [chest/total body computed tomography (CT), positron emission tomography (PET)-CT, endobronchial ultrasound (EBUS) and preoperative biopsies] and pulmonary function test. Each case was discussed in a multidisciplinary tumor board, where—according to the stage of disease and patient’s comorbidities—the indication to a possible preoperative treatment was given. The patients underwent 3 cycles of platinum-based chemotherapy and then revaluated for surgery. The re-staging for patients after neoadjuvant chemotherapy was carried out mainly by PET-CT scan and when necessary also preoperative biopsies with EBUS.
Intraoperative results and short-term postoperative outcomes in terms of complications, duration of chest tube, intensive care unit (ICU) admission, hospital stay, mortality and overall survival were recorded and evaluated.
Surgical technique
The same surgical team performed all procedures. The surgery was performed under general anesthesia and single-lung ventilation. The patients were placed in lateral decubitus with their arms flexed and stretched towards their head (13). A single 3–4 cm muscle-sparing incision was made on the midaxillary line in the IV or V intercostal space according to which lobe to be operated on. A wound protector was placed in all cases. A 10 mm 30° thoracoscope and special thoracoscopic instruments were used. All specimens were removed after putting in an endobag. The pain management was started with an extrapleural paravertebral intercostal nerve block as described previously (13). Only one chest drain (24 Fr) was placed at the end of the operation.
Postoperative management
All patients were treated according to our fast track protocol (14). The postoperative management was started with forced mobilization and respiratory physiotherapy in the immediate postoperative period.
The first chest X-ray was performed 2 hours after the operation and on the first postoperative day as well as before the expected chest tube removal, that was done when there was no air leak sign and the secretion was below 200–300 mL within 24 h.
Statistical analysis
Categorical variables are reported as n (%). Continuous variables are expressed as mean ± standard deviation (SD). Categorical variables were compared by Fischer’s exact test and continuous variables by independent sample Student’s t-test. Any possible correlations between outcome variables were explored by Pearson’s sample correlation r. Kaplan-Meier method was used for evaluating overall survival of the series; the comparison of two survival curves was done using the log-rank test. A P value less than 0.05 was considered statistically significant.
Statistical analysis was performed using IBM SPSS Statistics for Macintosh, Version 25.00 (Armonk, NY, USA).
Results
The mean age of 154 patients was 67.51±10.63 years and the 65.6% (101 patients) of the series were males.
The main demographic and preoperative clinical characteristics of the two groups (the 136 patients underwent surgery and the 18 patients underwent neoadjuvant chemotherapy (ChT + surgery) are reported in Table 1. The two groups resulted comparable in terms of age, gender, comorbidities and preoperative lung functionality.

Full table
The group of patients underwent neoadjuvant therapy presented in the 33% (6 patients) of cases a clinical stage IIIA, in 17% (3 patients) a cIIIb stage and in 50% (9 patients) a cIV stage (oligo metastatic patients). In the 72% of cases (13 patients) the diagnosis was an adenocarcinoma, in 28% (5 patients) a squamous cell carcinoma.
The mean operative time was overlapping in both groups: 248.97±118.17 min in surgery group and 287.17±94.13 min in ChT + surgery group, P=0.190.
No difference was also recorded between the main types of major lung resections performed in the two groups (P=0.487) and in the number of lymph nodes retrieved during lymphadenectomy (20.14±10.73 in surgery group vs. 21.61±7.37 in ChT + surgery, P=0.133, see Table 2).

Full table
There was no conversion in ChT + surgery group, while there was 8 cases (5.6%) in the surgery group (P=0.291). The intraoperative mortality was null in both groups.
The rate of ICU admission was the same in both groups (P=0.764, see Table 3).

Full table
A trend toward statistical significance was found in hospital stay, that was longer in ChT + surgery group compared to the other (P=0.061, see Table 3).
The 30-day mortality was null for ChT + surgery group and 1.5% for surgery group (2 patients dead for ARDS, P=0.605).
The incidence of complications was the same in both groups (Table 3) and no correlation was found with any possible risk factor evaluated (age, gender, comorbidities, type of resection, histology, etc.). The types of complications in ChT + surgery group were parenchymal fistula in 4 cases, pneumonia in 1 case and lung torsion in another one.
Among minor complications, the incidence of parenchymal fistula (causing a prolonged air-leakage) was significantly higher in the 18 patients underwent chemotherapy [4 (22.2%) vs. 7 (5.1%) cases, respectively, P=0.013). Consequently, in the same group, a higher incidence of reoperation rate (3 cases for re-checking aerostasis and 1 case for lung torsion) was also found (22.2% vs. 3.7%, P=0.007).
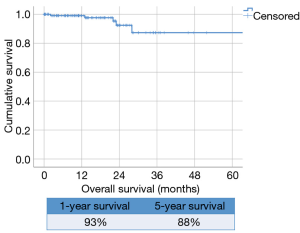
The overall survival of the whole series was 93% at 1 year follow-up and 88% at 5-year (Figure 1).
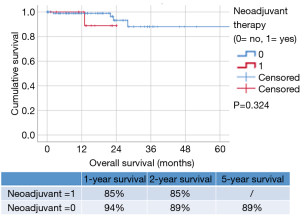
Comparing the survival between the two groups (Figure 2), the 1- and 2-year survival in only surgery group was 94% and 89% respectively vs. 85% and 85% in ChT + surgery, without any significant difference (P=0.324).
Discussion
In the last 20 years the role of the VATS has been more and more established for patients with early-stage NSCLC (15,16). The advantages of VATS towards thoracotomy have been proven by many studies as it allows faster recovery by reducing post-operative pain, preserving lung function allowing a reduction in the length of hospitalization time and a faster access to adjuvant treatments (17,18). Although the advantages of the minimally-invasive approach were recognized, it was initially considered contraindicated to treat advanced stage patients (stage IIIA) who had received neoadjuvant chemotherapy through VATS surgery (19). Neoadjuvant therapy causes the onset of pleural adhesions making the determination of the cleavage plans more complicated, favors vascular fragility increasing the difficulty of hilar and mediastinal dissection and making surgery a challenge even for an experienced surgeon (20,21). In the last years, due to the improvement of the surgical techniques and to the technological progress in the thoracoscopic field, this indication has changed (9). Several studies have shown that VATS lobectomy in patients with locally advanced NSCLC is safe and feasible and does not appear to compromise the oncologic outcomes confirming also the advantages against open thoracotomy (11,12,21,22). Few studies have shown how the VATS approach, when technically possible, was always preferable to open surgery but no comparative studies have been carried out between neoadjuvant- and non-neoadjuvant-treated patients who then underwent the same minimally-invasive technique.
In the best of our knowledge, this is the first work in literature, describing the short-term results of Uniportal VATS for anatomical lung resection for NSCLC performed with or without induction chemotherapy. In particular from June 2012 to September 2017, 18 patients in the ChT + surgery group (stage IIIA, IIIB, and IV) were evaluated and compared to 136 only surgery group.
According to the literature, patients undergoing neoadjuvant therapy face more complications after surgery than others. The rate of specific complications like cardiovascular diseases (38.8% vs. 30.9%) and respiratory diseases (44.4% vs. 30.1%) are higher in patients who have undergone induction therapy (11).
In our study, the length of operation was similar in the induction therapy group and surgery group (287.17±94.13 vs. 248.97±118.17 min, P=0.190). Probably the longer operative times and the prolonged duration of pleural drainage were due to the radical lymphadenectomy performed on different stations according to our standard, with a number of lymph nodes removed higher than this reported by others. In our series, the average number of lymph nodes removed was 21.61±7.37, compared to other studies (12,21,23).
Tissue edema and tissue inflammation induced by the neoadjuvant treatment caused a greater production of pleural effusion which resulted in the positioning of a pleural drainage for a longer period in ChT + surgery group (7.59±6.31 days). The chest tube duration in our group was more prolonged than others reported for pure VATS procedures in literature (12,21,23).
In concordance with other studies (24) we registered a greater risk of prolonged air leakage (4/18, 22.2% vs. 7/136, 5.1%) in ChT + surgery group, probably caused by inflammatory fibrosis, similar to those reported by other groups (12,21).
In 3 cases, it was necessary to revise the aerostasis surgically. We registered only one major complication (1/18, 5.55%, lung torsion) that required a reoperation and this was less than what reported in other studies following neoadjuvant therapy (12,21,25) or in multiportal VATS without induction therapy (16,26).
In the ChT + surgery group we did not register conversion to open thoracotomy [0% vs. 8/136 (5.9%) in the surgery group] and this was less than data reported in other series following neoadjuvant therapy (12,21,25).
The 30-day mortality rate in the ChT + surgery group was 0% (0/18) and 1.5% (2/136) in surgery group. This result was similar to those reported by other studies (21,25).
However, our study had some limitations: it was a monocentric and a retrospective one, involving a quite small sample of patients after neoadjuvant therapy. Furthermore, there was no comparison group of open surgery. Large and randomized studies would be needed for evaluating results on Uniportal VATS treatment of locally advanced NSCLC following neoadjuvant therapy and for validating the long-term oncologic outcomes.
In conclusion, from our preliminary results, uniportal VATS seems to be a quite safe and feasible technique for the treatment of locally advanced NSCLC following neoadjuvant therapy. Further corroborations are claimed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was evaluated by the Institutional Review Board (IRB) of Charité-Universitätsmedizin in Berlin and, as this was a retrospective review for service evaluation and there was no modification in patients’ care (no prospective randomized study), we did not need the final ethical approval of our IRB. All patients provided an informed consent for the treatment of their clinical data.
References
- Bezjak A, Temin S, Franklin G, et al. Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. J Clin Oncol 2015;33:2100-5. [Crossref] [PubMed]
- Gao SJ, Corso CD, Wang EH, et al. Timing of Surgery after Neoadjuvant Chemoradiation in Locally Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:314-22. [Crossref] [PubMed]
- Lewis J, Gillaspie EA, Osmundson EC, et al. Before or After: Evolving Neoadjuvant Approaches to Locally Advanced Non-Small Cell Lung Cancer. Front Oncol 2018;8:5. [Crossref] [PubMed]
- NSCLC Meta-analysis Collaborative Group. NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet (London, England) 2014;383:1561-71. [Crossref] [PubMed]
- Kim AW, Liptay MJ, Bonomi P, et al. Neoadjuvant chemoradiation for clinically advanced non-small cell lung cancer: an analysis of 233 patients. Ann Thorac Surg 2011;92:233-41. [Crossref] [PubMed]
- Mollberg NM, Mulligan MS. Video-assisted thoracoscopic (VATS) lobectomy after induction therapy. Thorac Surg Clin 2014;24:465-70. [Crossref] [PubMed]
- Kamel MK, Nasar A, Stiles BM, et al. Video-Assisted Thoracoscopic Lobectomy Is the Preferred Approach Following Induction Chemotherapy. J Laparoendosc Adv Surg Tech A 2017;27:495-500. [Crossref] [PubMed]
- Yang CF, Meyerhoff RR, Mayne NR, et al. Long-term survival following open versus thoracoscopic lobectomy after preoperative chemotherapy for non-small cell lung cancer. Eur J Cardiothorac Surg 2016;49:1615-23. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [PubMed]
- Abouarab AA, Rahouma M, Kamel M, et al. Single Versus Multi-Incisional Video-Assisted Thoracic Surgery: A Systematic Review and Meta-analysis. J Laparoendosc Adv Surg Tech A 2018;28:174-85. [Crossref] [PubMed]
- Yang CF, Meyerhoff RR, Mayne NR, et al. Long-term survival following open versus thoracoscopic lobectomy after preoperative chemotherapy for non-small cell lung cancer. Eur J Cardiothorac Surg 2016;49:1615-23. [Crossref] [PubMed]
- Jeon YJ, Choi YS, Lee KJ, et al. Outcomes of Pulmonary Resection and Mediastinal Node Dissection by Video-Assisted Thoracoscopic Surgery Following Neoadjuvant Chemoradiation Therapy for Stage IIIA N2 Non-Small Cell Lung Cancer. Korean J Thorac Cardiovasc Surg 2018;51:29-34. [Crossref] [PubMed]
- Ismail M, Swierzy M, Nachira D, et al. Uniportal video-assisted thoracic surgery for major lung resections: pitfalls, tips and tricks. J Thorac Dis 2017;9:885-97. [Crossref] [PubMed]
- Ismail M, Swierzy M, Nachira D, et al. Fast-Tracking Patients Through the Diagnostic and Therapeutic Pathways of Intrathoracic Conditions: The Role of Uniportal Video-Assisted Thoracic Surgery. Thorac Surg Clin 2017;27:425-30. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5. [Crossref] [PubMed]
- Hanna WC, de Valence M, Atenafu EG, et al. Is videoassisted lobectomy for non-small-cell lung cancer oncologically equivalent to open lobectomy? Eur J Cardiothorac Surg 2013;43:1121-5. [Crossref] [PubMed]
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [Crossref] [PubMed]
- Yang X, Wang S, Qu J. Video-assisted thoracic surgery (VATS) compares favorably with thoracotomy for the treatment of lung cancer: a five-year outcome comparison. World J Surg 2009;33:1857-61. [Crossref] [PubMed]
- Shaw JP, Dembitzer FR, Wisnivesky JP, et al. Video-assisted thoracoscopic lobectomy: state of the art and future directions. Ann Thorac Surg 2008;85:S705-9. [Crossref] [PubMed]
- Huang J, Xu X, Chen H, et al. Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer. J Thorac Dis 2013;5:S267-73. [PubMed]
- Kamel MK, Nasar A, Stiles BM, et al. Video-Assisted Thoracoscopic Lobectomy Is the Preferred Approach Following Induction Chemotherapy. J Laparoendosc Adv Surg Tech A 2017;27:495-500. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Venuta F, Anile M, Diso D, et al. Operative complications and early mortality after induction therapy for lung cancer. Eur J Cardiothorac Surg 2007;31:714-7. [Crossref] [PubMed]
- Park BJ, Yang HX, Woo KM, et al. Minimally invasive (robotic assisted thoracic surgery and videoassisted thoracic surgery) lobectomy for the treatment of locally advanced non-small cell lung cancer. J Thorac Dis 2016;8:S406-13. [Crossref] [PubMed]
- Kim K, Kim HK, Park JS, et al. Video-assisted thoracic surgery lobectomy: single institutional experience with 704 cases. Ann Thorac Surg 2010;89:S2118-22. [Crossref] [PubMed]