Functional mitral regurgitation: an overview for surgical management framework
The clinical problem
Functional mitral regurgitation (FMR) is one of the most common complications following a myocardial infarction. Randomized controlled trials to determine the optimal surgical approach have been performed (1-6), along with investigations aimed at evaluating alternative and emerging treatment strategies (7-11). Amongst patients with a history of myocardial infarction, approximately 50% and 10% develop moderate and moderate-to-severe mitral regurgitation (MR), respectively (2,4,10,11). The occurrence of FMR is responsible for more deaths and complications than all other potential consequences of a myocardial infarction combined (12). FMR results from a dysfunction of the valvular and subvalvular apparatus of the mitral valve (MV) due to displacement of one or both papillary muscles (PM) after a myocardial infarction, which leads to MV leaflet tethering and incomplete systolic MV closure. Given the complexity of its pathogenesis, a solution to correct the valvular and subvalvular dysfunction, along with the left ventricular (LV) geometric distortion associated with ischaemic mitral regurgitation (IMR), has not yet been elucidated (6,13,14).
Geometric abnormalities and tethering of any or all of the segments of the MV cause FMR by creating a so-called “vector-dependent” MV dysfunction related to the direction of the displacement vectors of the PMs. Evidence suggests that the pattern of the geometric abnormalities in FMR is variable, possibly reflecting heterogeneity in the imbalance of the tethering and closing forces, and in the biomechanical features of the valve, which may have important implications in the clinical management of this entity (8). The present article provides a review of the basic principles, recent advances, and recommendations for the surgical treatment of FMR.
Pathophysiology and effect of therapy
The prevalence of FMR ranges from 1.6 to 2.8 million in the United States (15). As mentioned, moderate or greater FMR develops progressively in more than half of patients after a myocardial infarction, and is sustained by varying degrees of disequilibrium between tethering and closing forces (16). Indeed, patients who develop heart failure from FMR also tend to have a dilated and remodeled LV, which results in PM displacement. The prevalence of LV remodeling and geometric perturbation, global dilatation, and systolic dysfunction increases with the severity of MR and are considered factors involved in worsening of the disease process (17). The extent of LV remodeling is also affected by the direction and severity of vector displacement of the PMs, regional LV dysfunction, and the degree of PM dyssynchrony (7,8,18,19). The development and perpetuation of FMR has been also attributed to reduced MV closing forces. The evidence supporting the role of closing forces in FMR includes the following observations: a reduction of LV contractility and global LV dyssynchrony is prevalent in patients with less than severe FMR, altered mitral annular systolic contraction is observed in patients with moderate leaflet tethering, and PM dyssynchrony persists in patients who received restrictive annuloplasty MV repair (20).
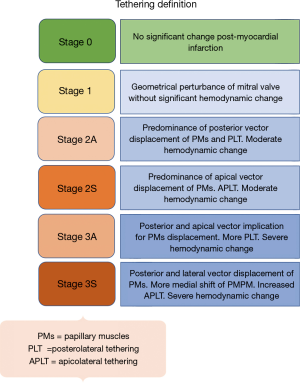
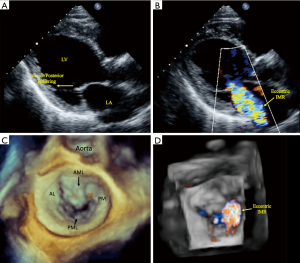
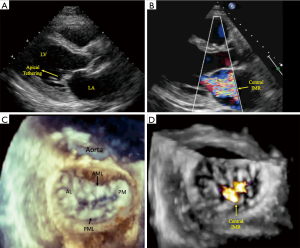
The interplay between reduced MV closing forces, and LV and PM dyssynchrony, has been characterized by: (I) geometrical MV abnormalities in leaflet attachments and tethering that is due to a delayed regional LV mechanical activation; (II) LV dyssynchrony itself may negatively impact systolic function, which further reduces the valvular closing forces; and (III) abnormal atrioventricular relaxation during contraction cycles causes higher left atrial pressure resulting in a positive pressure gradient within the LV chamber, affecting the mechanism of MV closure. Importantly, there is also significant dilatation and flattening of the MV annulus in patients with FMR, as well as a loss of the normal systolic folding and contraction mechanics. These characteristics worsen with disease chronicity, and it is debated as to whether they are a cause or effect of FMR. Classification schemes based on the patterns of MV leaflet tethering and closing force abnormalities, and grading of the severity mitral valvular and subvalvular apparatus dysfunction in FMR have not been established. However, differences in the vectorial displacement of the PMs have been reported, and echocardiography-based studies have identified two types of restricted systolic leaflet motion according to the tethering shape: the asymmetrical pattern with predominant posterior tethering of both leaflets that is most often observed with an inferior/posterior myocardial infarction (Figure 1: class 2A and 3A), and the symmetrical pattern with predominant apical tethering seen most commonly with anterior myocardial infarctions (Figure 1: class 2S and 3S) (20-23). Our group has successfully reproduced these patterns of FMR utilizing biomechanical models, with the hope that it may facilitate preoperative planning and postoperative management (8). The appearance of one of the two different forms of tethering depends on the relationship between the three tethering vectors observed in FMR: posterior, apical, and lateral (21-23). It is important to note that displacement of one of the PMs exerts a traction and tethering effect on both MV leaflet. In the asymmetric type, the posterior leaflet is moved more posteriorly than apically due to its parallel position in respect to the posterior LV wall. The restriction of the posterior leaflet leads to its malposition with the anterior leaflet, causing them to be in different planes during systole and resulting in asymmetric tethering and an eccentric mitral regurgitant jet (Figure 1: class 2A and 3A, Figure 2). Conversely, in the symmetrical type there is a combination of apical and medio-lateral vectorial tethering, as well as a more displaced coaptation point. The regurgitant jet is usually located centrally, and its direction reflects the equal involvement of the systolic motion in both leaflets (Figure 1: class 2S and 3S, Figure 3).
Clinical evidence
The initial diagnosis of FMR is made with the use of transthoracic echocardiography (TTE) (Figures 2,3). Although TTE is usually adequate in assessing the geometry of the valvular and subvalvular apparatus of the MV, a precise evaluation of tenting height, anterior-posterior mitral annular diameter, and interpapillary muscle distance (end-systole and end-diastole) measurements may be needed, in which case either a computed tomographic (CT) or magnetic resonance (MRI) imaging may be considered for a comprehensive assessment of the MV pathology. When contraindications to CT or MRI are present, transesophageal echocardiography can be performed. MRI is preferable to CT for serial surveillance, since it is not associated with radiation exposure. Yearly echocardiographic surveillance with TTE may be considered in patients with moderate FMR that do not require coronary revascularization, and who do not have an indication for MV surgery (24). If ongoing imaging surveillance, by means of echocardiography, CT, or MRI, reveals increasing MR or LV remodeling, then surgical management may be considered (25,26). In patients who have moderate to severe FMR with significant coronary artery disease, combined revascularization and mitral surgery should be offered (27,28).
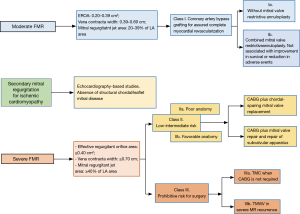
If there are concomitant indications for either valvular and/or coronary surgery, then a tailored surgical approach will be required, dictated largely by the ischemic MV pattern, perioperative risk, and surgeon and center experience. In patients with higher degrees of MV leaflet tethering and LV remodeling, the surgical options include MV replacement or repair (29) (Tables 1,2). In the latter case, restrictive mitral annuloplasty with procedures to correct subvalvular apparatus alterations has been increasingly advocated (5,6,30-34). Figure 4 depicts a decisional algorithm in FMR according to the revised American Association for Thoracic Surgery consensus guidelines (29).
Full table
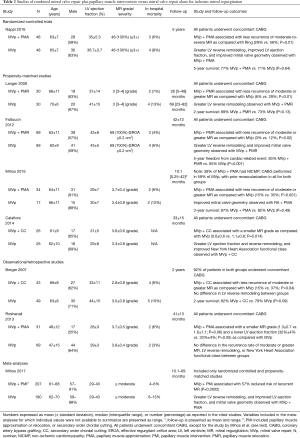
Full table
Clinical use
The most commonly recommended surgery for patients with moderate or severe FMR are MV repair or chordal-sparing replacement, but a lack of conclusive evidence in favor of one or the other technique has left the choice largely to the surgeon’s preference and expertise. Several randomized and observational studies have found that restrictive MV repair is associated with lower perioperative mortality but has high rate of MR recurrence, which is cited at 30% to 60% at mid-term follow-up (1,2). Undersizing valve repair is preferentially performed with closed rings, often with predetermined geometry, compared to partial ring or band. Conversely, replacement provides better long-term correction with a lower risk of MR recurrence and repeat surgery but has higher perioperative morbidity. In a recent meta-analysis was reported a rate of death at 35% higher in the replacement patients than in the repair subjects. This relative long-term risk has been attributed to the fact that patients undergoing mitral-valve replacement tend to be older and have more coexisting illnesses than those undergoing repair (35). When performing replacement of the MV, complete preservation of the subvalvular apparatus is recommended in order to avoid further dilatation of the LV chamber related to post myocardial infarction remodeling and improve peri-operative outcomes. The MV repair technique most commonly performed is a restrictive annuloplasty with the use of a rigid or semirigid ring to downsize the annulus diameter. Combined restrictive annuloplasty and subvalvular procedures directly addressing PM displacement and leaflet tethering have also been successfully performed (5,10,30-34). Procedures involving the PMs require knowledge of their anatomy and blood flow distribution, as well as recognition of the different divisions of PMs and anatomical variants. Two main procedures are performed in this context: PM approximation or “sling”, and PM relocation. The PMs anatomically classified as type I and II are approximated using a CV-4 Gore-Tex suture placed at the head of each PM. In type III, IV or V PMs, their approximation is performed with a 4-mm Gore-Tex tube encircling the bodies of each PM, which are then drawn together. In the presence of two independent posteromedial papillary muscle (PMPM) heads, both are included in the approximation to minimize MV tethering. In the relocation technique the PMs are fixed to the annular trigones, with the anterolateral papillary muscle (ALPM) to anterior and PMPM to posterior trigone, respectively. Of note, tethering by the secondary order (“strut”) chordae from the ALPM to the anterior leaflet is responsible for the development of the “seagull sign” on echocardiography. The achievement of the target interpapillary distance and the effectiveness of the procedure in resolving MR are confirmed with intraoperative transesophageal echocardiography. Attempts have been made to achieve the benefits of MV repair or replacement with the use of less invasive methods. Generally, patients undergoing surgery for FMR require concomitant coronary artery revascularization. Hybrid surgical and percutaneous revascularization performed with MV surgery have been reported (36). Additionally, approaches involving percutaneous revascularization in combination with minimally invasive access valve surgery to avoid median sternotomy have been described with encouraging results (37).
Results from randomized controlled trials and observational studies
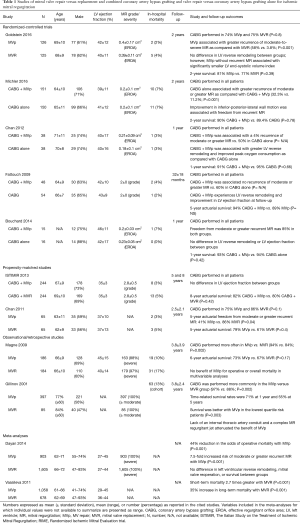
The results of the Cardiothoracic Surgical Trials Network (CTSN) randomized studies (Table 1) advanced the knowledge of the outcomes of surgery for the management of FMR. In one trial, 251 one patients with severe IMR were allocated to receive MV repair with a restrictive annuloplasty or chordal-sparing replacement (1,2). When comparing MV repair versus replacement at 2-year follow-up, there was no difference in the extent of LV reverse remodeling, LV ejection fraction or rate of death (19.0% vs. 23.2%). However, patients undergoing MV repair had a significantly higher incidence of recurrent MR (defined as moderate or greater) when compared with replacement (58% vs. 3.8%). Chan et al., reported similar findings, in a propensity-matched analysis of 130 patients in which the 5-year actuarial freedom from moderate or greater recurrent MR was 41% for MV repair versus 86% for replacement (38). Interestingly, echocardiographic evidence from the CTSN trial showed that in patients receiving MV repair that did not experience recurrent MR, LV reverse remodeling was significantly improved when compared with those who had recurrence. While analysis of these subgroups was not performed, it is important to note that late freedom from recurrent MR after MV repair for IMR is approximately 90% in LV reverse remodeling “responders”, which is defined as a reduction in the LV end-systolic volume index >15%. A second CTSN randomized trial evaluated 301 patients with moderate FMR, comparing those who received coronary artery bypass grafting (CABG) alone with patients who underwent combined CABG plus MV repair with a restrictive annuloplasty (3,4). While patients undergoing combined CABG plus MV repair had significantly less recurrent MR than CABG alone at 2-year follow-up (11.2% vs. 32.3%), there was no difference in LV reverse remodeling or rate of death (10% vs. 10.6%) and an increased incidence of adverse neurologic events (5.5% vs. 1.7%) and supraventricular arrhythmias. Similar outcomes were reported in three smaller randomized studies, including the RIME (Randomized Ischemic Mitral Evaluation) trial, in which combined CABG plus MV repair resulted in less recurrence of MR when compared with CABG alone at early to mid-term follow-up (0–15% vs. 15–60%) (39). However, this did not translate into a benefit in clinical outcomes. Finally, long-term follow-up data at 8 years from the ISTIMIR (The Italian Study on the Treatment of Ischemic Mitral Regurgitation) propensity-matched study found similar LV function and survival between a strategy of CABG plus MV repair versus CABG alone (40). Important limitations to the data regarding the efficacy of MV repair in FMR are centered on valvular anatomy and the technical aspects of the repair itself. Firstly, a restrictive annuloplasty addresses annular dilatation as the sole mechanism of FMR, when in fact the underlying substrate is LV dilatation and dysfunction leading to PM displacement and leaflet tethering. Indeed, the annular size may exceed more than 1.5 times its normal dimension prior to the development of FMR. Secondly, with a restrictive annuloplasty the fibrous posterior annulus is displaced anteriorly, which increases the annulo-papillary muscle distance and further tethers and restricts posterior mitral leaflet motion. Increased myocardial stress is also observed in the lateral LV wall, and these factors contribute to the high MR recurrence rates observed with MV repair. Thirdly, the hemodynamic MR burden may have been underestimated in these studies. An effective regurgitant orifice area of 0.2 cm2 has been associated with increased mortality in patients with FMR, and this cutoff was exceeded in most of the trials, particularly in the severe FMR CTSN trial where it measured approximately 0.4 cm2 in both the repair and replacement groups. Finally, the sample sizes limited the statistical power to evaluate the effects of surgical strategy on mortality, with reliance on surrogate clinical and echocardiographic endpoints.
The major clinical trial comparing MV repair versus replacement was limited because it involved a consistent number of patients who did not received CABG (26.2%) (2) and patients who underwent mitral-valve replacement tended to be older as reported by Milano et al. (41). The majority of the currently available randomized controlled or not-randomized evidences on IMR are based on small sample sizes and concomitant CABG procedures, the only interventions actually able to provide the necessary improvement of regional and global LV function to correct IMR, are not equally distributed among the subgroups. Despite the American College of Cardiology/American Heart Association and European Society of Cardiology/European Association for Cardiothoracic Surgery guidelines have been recommending surgical treatment of severe IMR for a long time, the debate still cannot reach a standing point as differences in the baseline characteristics of the patients harness the statistical power of the results. Indeed, it has been more than 40 years since the first FMR papers were published and now more than 50 papers are added each year under the key words “Surgery of Moderate or Severe IMR”. Despite this abundant and growing body of literature, particular risk factors that are unknown or unmeasured cannot adjust for the differences in baseline. Therefore, some studies have shown no differences in short- and long-term outcomes between repair and replacement groups, although the majority of studies favoring repair as emerged in work of Thourani et al. and Micovic et al. (42,43).
Results from randomized controlled trials and propensity score analysis: MV repair versus combined valvular and subvalvular repair
First described by Hvass, Kron and Rama and colleagues, a combined MV and subvalvular repair for FMR has been utilized with the aim of improving repair durability and decreasing the incidence of recurrent MR (5,18,30-33,44-46). Fattouch et al. reported the clinical and echocardiographic outcomes of PM relocation combined with MV repair using a non-restrictive mitral annuloplasty in a propensity-matched analysis of 138 patients with severe FMR (33). PM relocation plus MV repair was associated with a significantly smaller LV end-diastolic diameter, less recurrence of MR (3% vs. 12%), and a considerable improvement in MV geometry, when compared with MV repair alone at 42-month follow-up (47). Similar outcomes with combined PM relocation and MV repair for severe FMR were reported in a 60-patient propensity-matched study by Langer et al. (48), in which recurrent MR was observed in 6% of the combined PM relocation and MV repair patients vs. 29% of the MV repair alone group at mid-term follow-up (Table 2). In the only randomized trial evaluating the efficacy of subvalvular intervention in FMR, Nappi et al., allocated 96 patients to receive either MV repair with a restrictive annuloplasty alone or in combination with PM approximation. At 5-year follow-up, PM approximation in addition to MV repair resulted in a significantly smaller LV end-diastolic diameter, a greater LV ejection fraction, less recurrence of MR (27.9% vs. 55%), and improved MV geometry, with a similar survival rate (77.1% vs. 70.8%) when compared with MV repair alone (5,6). A multi-center propensity matched analysis of MV repair versus combined MV repair and PM approximation in the setting of ischemic or non-ischemic cardiomyopathy also revealed a lower prevalence of recurrent MR (15% vs. 35%) and improved MV geometry at early follow-up with the addition of PM approximation, with no difference observed between the types of cardiomyopathic substrate (49). Finally, a 2017 meta-analysis of five studies, which included 397 patients undergoing MV repair alone versus in combination with a subvalvular intervention, suggested the superiority of a combined valvular and subvalvular repair with regards to reduced risk of recurrent moderate or greater MR, MV geometry, and LV reverse remodeling (10). A subanalysis of the randomized trial by Nappi et al., demonstrated that PM approximation was beneficial in both symmetrical and asymmetrical tethering patterns of FMR by primarily correcting the posterior and lateral displacement of the PMs. PM approximation improved LV remodeling and MR recurrence rate in both tethering subgroups, but was also associated with a reduced risk of MV reoperation in patients with symmetric tethering, when compared with MV repair alone (0% vs. 16.7%) (6). These data are in accord with studies from Gelsomino et al., which highlighted that symmetric tethering is a negative predictor of LV reverse remodeling after annuloplasty MV repair, and that this condition requires a more comprehensive intervention to restore LV and subvalvular geometry (20,23,50-52). As performed in the trial, it is proposed that a 30% reduction in the interpapillary muscle distance by the approximation is sufficient to restore an adequate LV geometry and favorable redistribution of forces within the valvular and subvalvular apparatus. The reinforcement of the posterior annulus by means of a double row of overlapping sutures may also be beneficial to reduce the tension on the portion of the annulus experiencing the greatest stress (53,54). As stated earlier, the small sample sizes are important limitations in the studies of combined MV and subvalvular repair, owing in part to the difficulty in recruitment and randomization of patients with specific MV geometric or subvalvular apparatus abnormalities. Furthermore, most of the studies included a relatively heterogeneous population in terms of tethering pattern, regional wall motion abnormalities, and extent of coronary artery disease. Tethering and wall motion abnormality characteristics have important implications the selection of the PM intervention performed. For example, while the dysfunction and posterior PM displacement associated with an inferior wall myocardial infarction can be corrected by PM approximation, an overwhelming lateral displacement secondary to an anterior or lateral wall myocardial infarction may not be compensated by this approach, and these patients may be better suited with PM relocation stabilizing the PMs with fixation to the mitral trigones. Finally, the utilization of pre-operative echocardiography may help in identifying patients at high risk of MV repair failure, in whom the addition of a subvalvular intervention or performance of chordal-sparing replacement may be warranted. Several echocardiographic parameters reflecting the extent of MV tethering and LV geometric distortion and remodeling have been described, and are presented in Figure 1 (55,56).
IMR and coronary artery disease
Complete myocardial revascularization via concomitant CABG or staged percutaneous coronary intervention is an important and necessary step in the management of FMR In regards to surgical revascularization, the performance of CABG or CABG plus MV surgery are associated with a 44% and 31% reduction in long-term mortality, respectively, when compared with medical therapy alone (57).
The importance of myocardial revascularization and improvement in regional wall motion was also highlighted in the CTSN moderate IMR trial. It was shown that the percent improvement in global and in posterior-inferior-lateral wall motion was greater in patients who did not experience recurrent MR after CABG or CABG plus MV repair as compared with those who did have recurrent MR (3,4,58,59). Importantly, in patients with severe FMR and advanced post-ischemic LV remodeling, non-viable infarcted myocardium or PM dyssynchrony, coronary revascularization alone is typically insufficient to correct FMR, and concomitant chordal-sparing replacement or reparative strategies addressing both the annular and subvalvular components of the MV apparatus should be considered.
Unanswered questions and future directions
FMR is a complex pathological entity characterized by the presence of concomitant and interconnected abnormalities of the MV, subvalvular apparatus, and LV structure and function. A “holistic” approach to comprehensively address each of the components is required. For this purpose, a full understanding of the pathological changes and mechanisms of dysfunction is crucial. This way can satisfy a better understanding of the role of transcatheter MV therapy for treatment of ischemic versus degenerative cardiomyopathy in patients at prohibitive risk surgery. These evaluations are necessary without prejudice to the progressive affirmation of the transcatheter procedure for MV pathologies that is suitable in high risk patients with severe MR (60). Mathematical modeling of the MV along with the intriguing insight provided by finite element analysis aims to enable precise mapping of the pathogenesis of FMR in individual patients, and to analyze the potential changes induced by the application of different operative techniques (61). Questions regarding the indications and timing of MV intervention, the best suitable operative technique to be applied, and in cases of PM handling, the extent of the geometrical correction needed (48), still remain unanswered. Surely, in vitro and in silico studies may provide further insight; however, larger randomized clinical trials and targeted subgroup analyses with significant sample sizes are warranted in order on outcomes prediction and to enhance the comprehensive engineering and mathematical modeling of this disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare
References
- Acker MA, Parides MK, Perrault LP, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med 2014;370:23-32. [Crossref] [PubMed]
- Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med 2016;374:344-53. [Crossref] [PubMed]
- Smith PK, Puskas JD, Ascheim DD, et al. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2014;371:2178-88. [Crossref] [PubMed]
- Michler RE, Smith PK, Parides MK, et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N Engl J Med 2016;374:1932-41. [Crossref] [PubMed]
- Nappi F, Lusini M, Spadaccio C, et al. Papillary Muscle Approximation Versus Restrictive Annuloplasty Alone for Severe Ischemic Mitral Regurgitation. J Am Coll Cardiol 2016;67:2334-46. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Nenna A, et al. Is subvalvular repair worthwhile in severe ischemic mitral regurgitation? Subanalysis of the Papillary Muscle Approximation trial. J Thorac Cardiovasc Surg 2017;153:286-95.e2. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Fraldi M. Reply: Papillary Muscle Approximation Is an Anatomically Correct Repair for Ischemic Mitral Regurgitation. J Am Coll Cardiol 2016;68:1147-8. [Crossref] [PubMed]
- Nappi F, Spadaccio C. Biomechanics of failed ischemic mitral valve repair: discovering new frontiers. J Thorac Cardiovasc Surg 2017;154:832-3. [Crossref] [PubMed]
- Kron IL, Hung J, Overbey JR, et al. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2015;149:752-61.e1. [Crossref] [PubMed]
- Mihos CG, Xydas S, Yucel E, et al. Mitral valve repair and subvalvular intervention for secondary mitral regurgitation: a systematic review and meta-analysis of randomized controlled and propensity matched studies. J Thorac Dis 2017;9:S582-94. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Chello M, et al. Papillary muscle approximation in mitral valve repair for secondary MR. J Thorac Dis 2017;9:S635-9. [Crossref] [PubMed]
- Bursi F, Enriquez-Serrano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation 2005;111:295-301. [Crossref] [PubMed]
- Timek TA. Sub or snub: Is subvalvular repair worthwhile in severe ischemic mitral regurgitation? J Thorac Cardiovasc Surg 2017;153:296-7. [Crossref] [PubMed]
- Charles EJ, Kron IL. Repairing the mitral subvalvular apparatus: The new frontier. J Thorac Cardiovasc Surg 2017;153:284-5. [Crossref] [PubMed]
- de Marchena E, Badiye A, Robalino G, et al. Respective prevalence of the different carpentier classes of mitral regurgitation: a stepping stone for future therapeutic research and development. J Card Surg 2011;26:385-92. [Crossref] [PubMed]
- Otsuji Y, Levine RA, Takeuchi M, et al. Mechanism of ischemic mitral regurgitation. J Cardiol 2008;51:145-56. [Crossref] [PubMed]
- Rossi A, Dini FL, Faggiano P, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011;97:1675-80. [Crossref] [PubMed]
- Shudo Y, Matsumiya G, Sakaguchi T, et al. Assessment of changes in mitral valve configuration with multidetector computed tomography: impact of papillary muscle imbrication and ring annuloplasty. Circulation 2010;122:S29-36. [Crossref] [PubMed]
- Kalra K, Wang Q, McIver BV, et al. Temporal changes in interpapillary muscle dynamics as an active indicator of mitral valve and left ventricular interaction in ischemic mitral regurgitation. J Am Coll Cardiol 2014;64:1867-79. [Crossref] [PubMed]
- van Garsse L, Gelsomino S, Cheriex E, et al. Tethering symmetry reflects advanced left ventricular mechanical dyssynchrony in patients with ischemic mitral regurgitation undergoing restrictive mitral valve repair. Ann Thorac Surg 2012;94:1418-28. [Crossref] [PubMed]
- Zeng X, Nunes MC, Dent J, et al. Asymmetric versus symmetric tethering patterns in ischemic mitral regurgitation: geometric differences from three-dimensional transesophageal echocardiography. J Am Soc Echocardiogr 2014;27:367-75. [Crossref] [PubMed]
- Kim K, Kaji S, An Y, et al. Mechanism of asymmetric leaflet tethering in ischemic mitral regurgitation: 3D analysis with multislice CT. JACC Cardiovasc Imaging 2012;5:230-2. [Crossref] [PubMed]
- Agricola E, Oppizzi M, Maisano F, et al. Echocardiographic classification of chronic ischemic mitral regurgitation caused by restricted motion according to tethering pattern. Eur J Echocardiogr 2004;5:326-34. [Crossref] [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159-95. [Crossref] [PubMed]
- Lancellotti P, Moura L, Pierard LA, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307-32. [Crossref] [PubMed]
- Sundt TM. Surgery for ischemic mitral regurgitation. N Engl J Med 2014;371:2228-9. [Crossref] [PubMed]
- Perrault LP, Moskowitz AJ, Kron IL, et al. Optimal surgical management of severe ischemic mitral regurgitation: to repair or to replace? J Thorac Cardiovasc Surg 2012;143:1396-403. [Crossref] [PubMed]
- AATS Ischemic Mitral Regurgitation Consensus Guidelines Writing Committee, Kron IL, LaPar DJ, et al. 2016 update to The American Association for Thoracic Surgery (AATS) consensus guidelines: Ischemic mitral valve regurgitation. J Thorac Cardiovasc Surg 2017;153:e97-114. [Crossref] [PubMed]
- Rama A, Nappi F, Praschker BG, et al. Papillary muscle approximation for ischemic mitral valve regurgitation. J Card Surg 2008;23:733-5. [Crossref] [PubMed]
- Kron IL, Green GR, Cope JT. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. Ann Thorac Surg 2002;74:600-1. [Crossref] [PubMed]
- Hvass U, Tapia M, Baron F, et al. Papillary muscle sling: a new functional approach to mitral repair in patients with ischemic left ventricular dysfunction and functional mitral regurgitation. Ann Thorac Surg 2003;75:809-11. [Crossref] [PubMed]
- Fattouch K, Castrovinci S, Murana G, et al. Papillary muscle relocation and mitral annuloplasty in ischemic mitral valve regurgitation: midterm results. J Thorac Cardiovasc Surg 2014;148:1947-50. [Crossref] [PubMed]
- Langer F, Rodriguez F, Ortiz S, et al. Subvalvular repair: the key to repairing ischemic mitral regurgitation? Circulation 2005;112:I383-9. [PubMed]
- Vassileva CM, Boley T, Markwell S, et al. Meta-analysis of short-term and long-term survival following repair versus replacement for ischemic mitral regurgitation. Eur J Cardiothorac Surg 2011;39:295-303. [Crossref] [PubMed]
- Umakanthan R, Leacche M, Petracek MR, et al. Combined PCI and minimally invasive heart valve surgery for high-risk patients. Curr Treat Options Cardiovasc Med 2009;11:492-8. [Crossref] [PubMed]
- Santana O, Singla S, Mihos CG, et al. Outcomes of a Combined Approach of Percutaneous Coronary Revascularization and Cardiac Valve Surgery. Innovations (Phila) 2017;12:4-8. [Crossref] [PubMed]
- Chan V, Ruel M, Mesana TG. Mitral valve replacement is a viable alternative to mitral valve repair for ischemic mitral regurgitation: a case-matched study. Ann Thorac Surg 2011;92:1358-65; discussion 1365-6. [Crossref] [PubMed]
- Chan KM, Punjabi PP, Flather M, et al. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation 2012;126:2502-10. [Crossref] [PubMed]
- Lorusso R, Gelsomino S, Vizzardi E, et al. Mitral valve repair or replacement for ischemic mitral regurgitation? The Italian Study on the Treatment of Ischemic Mitral Regurgitation (ISTIMIR). J Thorac Cardiovasc Surg 2013;145:128-39; discussion 137-8. [Crossref] [PubMed]
- Milano CA, Daneshmand MA, Rankin JS, et al. Survival prognosis and surgical management of ischemic mitral regurgitation. Ann Thorac Surg 2008;86:735-44. [Crossref] [PubMed]
- Thourani VH, Weintraub WS, Guyton RA, et al. Outcomes and long-term survival for patients undergoing mitral valve repair versus replacement: effect of age and concomitant coronary artery bypass grafting. Circulation 2003;108:298-304. [Crossref] [PubMed]
- Micovic S, Milacic P, Otasevic P, et al. Comparison of valve annuloplasty and replacement for ischemic mitral valve incompetence. Heart Surg Forum 2008;11:E340-5. [Crossref] [PubMed]
- Fattouch K, Lancellotti P, Castrovinci S, et al. Papillary muscle relocation in conjunction with valve annuloplasty improve repair results in severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2012;143:1352-5. [Crossref] [PubMed]
- Szymanski C, Bel A, Cohen I, et al. Comprehensive annular and subvalvular repair of chronic ischemic mitral regurgitation improves long-term results with the least ventricular remodeling. Circulation 2012;126:2720-7. [Crossref] [PubMed]
- Fattouch K, Murana G, Castrovinci S, et al. The role of papillary muscle relocation in ischemic mitral valve regurgitation. Semin Thorac Cardiovasc Surg 2012;24:246-53. [Crossref] [PubMed]
- Mihos CG, Capoulade R, Yucel E, et al. Combined papillary muscle sling and ring annuloplasty for moderate-to-severe secondary mitral regurgitation. J Card Surg 2016;31:664-71. [Crossref] [PubMed]
- Langer F, Kunihara T, Hell K, et al. RING+STRING: Successful repair technique for ischemic mitral regurgitation with severe leaflet tethering. Circulation 2009;120:S85-91. [Crossref] [PubMed]
- Gelsomino S, Lorusso R, De Cicco G, et al. Five-year echocardiographic results of combined undersized mitral ring annuloplasty and coronary artery bypass grafting for chronic ischaemic mitral regurgitation. Eur Heart J 2008;29:231-40. [Crossref] [PubMed]
- Gelsomino S, Lorusso R, De Cicco G, et al. Does preoperative tethering symmetry affect left ventricular reverse remodeling after restrictive annuloplasty? Int J Cardiol 2010;141:182-91. [Crossref] [PubMed]
- Gelsomino S, Lorusso R, Caciolli S, et al. Insights on left ventricular and valvular mechanisms of recurrent ischemic mitral regurgitation after restrictive annuloplasty and coronary artery bypass grafting. J Thorac Cardiovasc Surg 2008;136:507-18. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Al-Attar N, et al. Downsizing annuloplasty in ischemic mitral regurgitation: double row overlapping suture to avoid ring disinsertion in valve repair. Surg Technol Int 2014;25:203-6. [PubMed]
- Nappi F, Spadaccio C, Chello M, et al. Double row of overlapping sutures for downsizing annuloplasty decreases the risk of residual regurgitation in ischaemic mitral valve repair. Eur J Cardiothorac Surg 2016;49:1182-7. [Crossref] [PubMed]
- Capoulade R, Zeng X, Overbey JR, et al. Impact of Left Ventricular to Mitral Valve Ring Mismatch on Recurrent Ischemic Mitral Regurgitation After Ring Annuloplasty. Circulation 2016;134:1247-56. [Crossref] [PubMed]
- Charles EJ, Kron IL. Data, not dogma, for ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2017;154:137-8. [Crossref] [PubMed]
- Castleberry AW, Williams JB, Daneshmand MA, et al. Surgical revascularization is associated with maximal survival in patients with ischemic mitral regurgitation: a 20-year experience. Circulation 2014;129:2547-56. [Crossref] [PubMed]
- Fattouch K, Guccione F, Sampognaro R, et al. POINT: Efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg 2009;138:278-85. [Crossref] [PubMed]
- Bouchard D, Jensen H, Carrier M, et al. Effect of systematic downsizing rigid ring annuloplasty in patients with moderate ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2014;147:1471-7. [Crossref] [PubMed]
- Feldman T, Kar S, Elmariah S, et al. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. J Am Coll Cardiol 2015;66:2844-54. [Crossref] [PubMed]
- Fraldi M, Spadaccio C, Mihos CG, et al. Analysing the reasons of failure of surgical mitral repair approaches-do we need to better think in biomechanics? J Thorac Dis 2017;9:S661-4. [Crossref] [PubMed]
- Roshanali F, Mandegar MH, Yousefnia MA, et al. A prospective study of predicting factors in ischemic mitral regurgitation recurrence after ring annuloplasty. Ann Thorac Surg 2007;84:745-9. [Crossref] [PubMed]


