Subxiphoid pneumonectomy: the new frontier?
Introduction
Over the last decade there has been a significant drive towards minimally invasive thoracic surgery. The reported benefits of video-assisted thoracic surgery (VATS) over open surgery include reduced postoperative pain, reduced postoperative complications, reduced length of hospital-stay and period of recovery to normal function (1-4). With increased experience, more advanced procedures are now reportedly performed by VATS in high volume centres, including sleeve and carinal resections (5,6).
Over the last decade, there have been several reports of pneumonectomy being performed by VATS in experienced centres (7-10). The majority of cases have been performed by multi-port VATS, although more recently, as for standard lobectomies (11,12), there has been evolution to uniportal VATS surgery as a means of potentially further reducing pain by utilising a single incision (13). A potential disadvantage of an intercostal VATS approach to pneumonectomy is the requirement to remove the lung through the intercostal space which may require a degree of rib spreading, cause intercostal nerve compression and damage, and in some series is achieved by taking the lung out in pieces which has potential oncological consequences, especially with regard to staging assessment and to pleural spread of malignant cells.
More recently, the subxiphoid-VATS approach has been described and is increasingly utilised for a range of thoracic operations including thymectomy, lobectomy, segmentectomies and resection of giant solitary fibrous tumours of the pleura (14-21). In this technique, a single vertical muscle-sparing incision is made in the subxiphoid space permitting thoracic surgery to be performed without requiring intercostal incisions. The specific potential advantages of this approach for pneumonectomy include reduced pain—both acute and chronic, in comparison to intercostal VATS surgery, and the ability to retrieve the lung whole from the pleural cavity without needing to spread ribs or run the risk of intercostal nerve injury.
Here we describe our technique for performing pneumonectomy by subxiphoid uniportal VATS, which has been successfully performed in three patients to date.
Operative technique
The operation is performed with the patient under general anaesthesia and double lumen endotracheal tube intubation to allow for selective lung ventilation. The patient is placed in a lateral decubitus position slightly rotated posteriorly in order to optimize exposure of the subxiphoid region. The operating surgeon is positioned in front of the patient and the assistant stands more caudally on the side being operated on to provide the optimal endoscopic view.
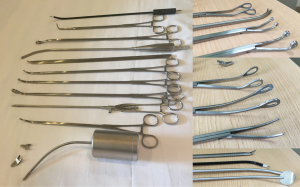
A 3–5 cm midline vertical incision is made below the sternocostal triangle to expose the xiphoid process. Dissection of the linea alba is performed and the pleural cavity was entered by blunt finger dissection above the level of the diaphragm. An Alexis soft tissue wound retractor (Applied Medical CA, USA) is positioned to create a space for instrument and a 30-degree 10 mm camera insertion (Karl Storz, Tuttlingen, Germany). Subxiphoid VATS instruments were used to perform the procedure (Figure 1 Shanghai Medical Instruments Group Ltd, Shanghai, China).
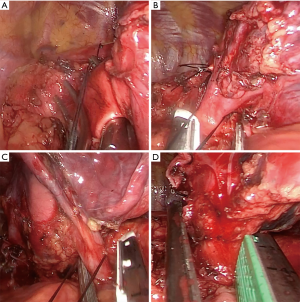
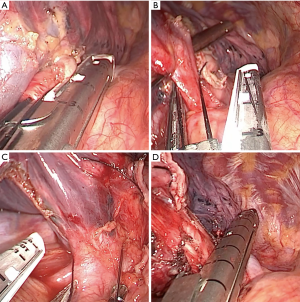
The pneumonectomy is performed as demonstrated in Figures 2,3 (a left sided pneumonectomy) and Figures 4,5 (a right sided pneumonectomy). The mediastinal pleura is divided to expose the hilum with diathermy. The superior pulmonary vein is dissected and a sling positioned in order to offer some retraction. The main pulmonary artery is then dissected, slung and divided with an Endo-GIA surgical stapler with vascular reload (Medtronic EndoGIA, MA, USA). Next, the previously prepared superior pulmonary vein is divided with a vascular reload. The inferior pulmonary ligament in then divided and inferior pulmonary vein dissected, slung and then divided with a vascular reload. Finally the main bronchus is dissected and divided with a green Endo-GIA stapler reload. The specimen was removed using an endoscopic retrieval bag (EndoBag, Covidien, Mansfield, MA, USA). It may be required to extend the skin incision to facilitate retrieval of the specimen. A full lymphadenectomy is performed. The bronchial stump is checked under water for air leak and a drain positioned. The subxiphoid incision site is then closed.


Results
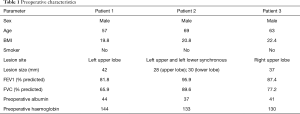
To date we have performed this procedure in three patients at the Shanghai Pulmonary Hospital, Shanghai, China. Preoperative characteristics of the three patients are summarised in Table 1.
Full table
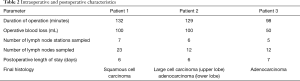
Intraoperative and postoperative characteristics are presented in Table 2. The mean duration of surgery was 119 minutes. Lymph node stations were accessible and a mean of six lymph node stations were sampled. There were no intraoperative complications encountered and no cases of conversion to multiport VATS or thoracotomy. No patient experienced an arrhythmia during the procedure. All patients made a good recovery from surgery and were discharged between the 6th and 7th post-operative day. Mean follow up is 181 days. There has been no mortality to date.
Full table
Discussion
This report demonstrates that the subxiphoid uniportal VATS approach can be safely and effectively utilised to perform a pneumonectomy.
Minimally invasive techniques are increasingly being utilised for advanced thoracic surgical procedures at experienced centres (5,6). In the case of pneumonectomy, there are now several reports describing the use of thoracoscopic techniques (7-10). The rationale to pursue a thoracoscopic approach are the reported benefits observed for other procedures such as lobectomies, where postoperative pain, length of hospital-stay and time to return to normal function have been demonstrated to be lower in comparison with open surgery (1-3).
Reports from animal model work in both pigs and dogs has suggested that VATS pneumonectomy results in a smaller incision, less acute-phase reaction, less stress and less pain compared with thoracotomy pneumonectomy suggesting that there will also be benefits to patients (24,25).
To date there has not been any randomised controlled trial comparing open versus VATS pneumonectomy allowing a robust comparison of clinical outcomes. The closest is a study from a US centre reporting their 11-year experience of performing thoracoscopic pneumonectomy (7). In this report, authors compared patient outcomes of 40 open and 50 VATS consecutive pneumonectomies. As this was not randomised patient characteristics varied between the groups. However, the incidence of postoperative complications, length of ICU and hospital stay and mortality were similar between the two groups. Notably, although in-hospital analgesic requirements were similar, there was a significant difference in the proportion pain free at 1-year (53% vs. 19%).
Another important consideration is the ability to achieve an equivalent oncological result. For VATS lobectomy oncological validity has been confirmed. Again, it is difficult to comment without randomised controlled data for VATS pneumonectomy, but the rate of locoregional recurrence and long-term survival were no different in the above study (7). A further study also examined the long term survival of patients after thoracoscopic pneumonectomy and found there to be no difference in survival when stratified for stage between their cohorts of patients who underwent thoracoscopic vs. open pneumonectomy (26).
It should be emphasised that pneumonectomy is only performed when it is confirmed that parenchymal-sparing alternative approaches are not feasible, for example sleeve resections. These will always be attempted in the first instance and thorough assessment made prior to proceeding with pneumonectomy. With increased experience, there are reports of these advanced parenchymal-sparing resections being performed by VATS, even uniportal, at our centre, confirming that adequate assessment can be made by VATS (5,6,27).
There is increasing experience of performing VATS pneumonectomy throughout the world. We feel that subxiphoid VATS offers the advantage of not having an entry into the pleural cavity that is bound by the ribs. Retrieval of the specimen can be challenging and depending on the size of the tumour may require extension of the incision in both approaches. In the case of standard VATS, there are reports of requiring rib spreading and the lung being retrieved in pieces. Furthermore, retrieval may result in compression of the intercostal nerves which can result in chest wall pain which may be chronic in some patients
More recently, the subxiphoid uniportal VATS technique has been described, avoiding any intercostal incision or instrumentation (14-21,28,29). Additionally, since the chest drains are not entering the thoracic cavity through intercostal spaces postoperatively, they do not impinge on the intercostal neurovascular bundle during mobilization of the patient. This has the potential benefit of allowing for early aggressive mobilization and clearance of secretions with the benefit of reducing the incidence of venous thromboembolism and lower respiratory tract infection and therefore possibly reduced length of hospital stay (30).
To date, there have been three studies comparing subxiphoid and uniportal VATS techniques. The first was in a dog model of pulmonary lobectomy where 19 dogs were divided into a subxiphoid and uniportal groups. This study concluded that the subxiphoid approach was comparable with the standard transthoracic approach for anatomic pulmonary lobectomy, in terms of feasibility and effectiveness based on their physiological assessments (31). Another study retrospectively reviewed cohorts of patients undergoing subxiphoid or standard VATS thymectomy. This study observed similar operative times for the two groups, but the amount and required duration of analgesia was significantly reduced in the subxiphoid VATS group (32). The third study is a randomised controlled trial randomised 43 patients to either subxiphoid or standard uniportal VATS bullectomy and pleurectomy for the management of pneumothorax. Postoperative pain scores were significantly lower in the subxiphoid group, although the operative time was longer in this group, perhaps reflecting lower experience (33). From these studies, it appears that although further work is necessary, that the subxiphoid VATS approach is associated with reduced post-operative pain. As such, there are increasing reports of the use of this technique in the literature for a growing range of thoracic procedures.
It should also be highlighted that stapler angles, which can be sometimes quite challenging with uniportal VATS are much more favourable with the subxiphoid approach. The subxiphoid VATS technique can be easily learnt by surgeons experienced in uniportal VATS. The camera views and instrument positioning can be more challenging than for conventional VATS but the necessary skills can be acquired over a short learning curve, particularly for minor procedures.
Limitations of the technique
Particularly in left sided procedures there can be compression of the heart by the instruments. This occasionally can result in arrhythmias and reduced cardiac output requiring an alternative approach. In our experience of subxiphoid lobectomy and segmentectomy, an intra-operative arrhythmia has been experienced in about 7% of cases, but in almost all cases was transient and mild and did not require changing technique. None of the patients in this series though experienced these problems. All patients have an arterial line and the blood pressure is monitored throughout the procedure. We do consider that cardiomyopathy and poor left ventricular function to be a contraindication to subxiphoid VATS surgery.
Access to some areas of the pleural cavity can be challenging with subxiphoid VATS. Access to the posterior hilum, lymph node station 7 and posterior pulmonary segments [2, 6, 8, 9] can be more challenging than with standard VATS. In fact, early in our experience with this technique we considered that these segmentectomies could not be performed. However, with increased experience and availability of bespoke subxiphoid instruments (Figure 1) these are now performed readily. In terms of pneumonectomy, the lymph node dissection can be performed easily following removal of the specimen. A key factor to being able to perform this surgery is expert assistance with the camera, as achieving satisfactory visualisation can be challenging initially.
In view of the above perceived benefits in terms of pain and our increasing experience with the subxiphoid VATS approach, the move to subxiphoid VATS pneumonectomy was without complication. From the perspective of performing a pneumonectomy, the ability to retrieve the whole in-tact specimen due to the absence of rigid boundaries of the incision, is an additional attraction of potential oncological significance. As described in this case series, subxiphoid uniportal VATS pneumonectomy is safe and feasible with several potential benefits that may result in faster patient recovery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Shanghai Pulmonary Hospital Ethical committee (number 002536) and informed consent was obtained from all patients.
References
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194-200. [Crossref] [PubMed]
- Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thorac Surg 2008;85:S719-28. [Crossref] [PubMed]
- Sugiura H, Morikawa T, Kaji M, et al. Long-term benefits for the quality of life after video-assisted thoracoscopic lobectomy in patients with lung cancer. Surg Laparosc Endosc Percutan Tech 1999;9:403-8. [Crossref] [PubMed]
- Pu Q, Ma L, Mei J, et al. Video-assisted thoracoscopic surgery versus posterolateral thoracotomy lobectomy: A more patient-friendly approach on postoperative pain, pulmonary function and shoulder function. Thorac Cancer 2013;4:84-9. [Crossref] [PubMed]
- Lyscov A, Obukhova T, Ryabova V, et al. Double-sleeve and carinal resections using the uniportal VATS technique: a single centre experience. J Thorac Dis 2016;8:S235-41. [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i6-16. [PubMed]
- Battoo A, Jahan A, Yang Z, et al. Thoracoscopic pneumonectomy: an 11-year experience. Chest 2014;146:1300-9. [Crossref] [PubMed]
- Kim AW, Fonseca AL, Boffa DJ, et al. Experience with thoracoscopic pneumonectomies at a single institution. Innovations (Phila) 2014;9:82-6; discussion 86. [Crossref] [PubMed]
- Chen HW, Du M. Video-assisted thoracoscopic pneumonectomy. J Thorac Dis 2015;7:764-6. [PubMed]
- Conlan AA, Sandor A. Total thoracoscopic pneumonectomy: indications and technical considerations. J Thorac Cardiovasc Surg 2003;126:2083-5. [Crossref] [PubMed]
- Ismail NA, Elsaegh M, Dunning J. Novel Techniques in Video-assisted Thoracic Surgery (VATS) Lobectomy. Surg Technol Int 2015;26:206-9. [PubMed]
- Gonzalez-Rivas D. VATS lobectomy: surgical evolution from conventional VATS to uniportal approach. ScientificWorldJournal 2012;2012. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Uniportal video-assisted thoracoscopic pneumonectomy. J Thorac Dis 2013;5 Suppl 3:S246-52. [PubMed]
- Aresu G, Jiang L, Bertolaccini L. Subxiphoid video-assisted major lung resections: the Believers' speech. J Thorac Dis 2017;9:E387-9. [Crossref] [PubMed]
- Aresu G, Weaver H, Wu L, et al. The Shanghai Pulmonary Hospital uniportal subxiphoid approach for lung segmentectomies. J Vis Surg 2016;2:172. [Crossref] [PubMed]
- Aresu G, Wu L, Lin L, et al. The Shanghai Pulmonary Hospital subxiphoid approach for lobectomies. J Vis Surg 2016;2:135. [Crossref] [PubMed]
- Fok M, Karunanantham J, Ali JM, et al. Subxiphoid approach for spontaneous bilateral pneumothorax: a case report. J Vis Surg 2017;3:146. [Crossref] [PubMed]
- Weaver H, Ali JM, Jiang L, et al. Uniportal subxiphoid video-assisted thoracoscopic approach for thymectomy: a case series. J Vis Surg 2017;3:169. [Crossref] [PubMed]
- Gonzalez-Rivas D, Lirio F, Sesma J, et al. Subxiphoid complex uniportal video-assisted major pulmonary resections. J Vis Surg 2017;3:93. [Crossref] [PubMed]
- Hatooka S, Shigematsu Y, Nakanishi M, et al. Subxiphoid approach for extracting a giant solitary fibrous tumour of the pleura. Interact Cardiovasc Thorac Surg 2017;25:834-5. [Crossref] [PubMed]
- Song N, Zhao DP, Jiang L, et al. Subxiphoid uniportal video-assisted thoracoscopic surgery (VATS) for lobectomy: a report of 105 cases. J Thorac Dis 2016;8:S251-7. [PubMed]
- Ali JM, Kaul P, Jiang L, et al. Subxiphoid VATS left pneumonectomy. Asvide 2018;5:640. Available online: http://www.asvide.com/article/view/26149
- Ali JM, Kaul P, Jiang L, et al. Subxiphoid VATS right pneumonectomy. Asvide 2018;5:641. Available online: http://www.asvide.com/article/view/26150
- Liu HF, Gao L, Liu T, et al. Comparison of acute phase reaction and postoperative stress in pigs undergoing video-assisted thoracoscopic versus thoracotomy pneumonectomy. Acta Vet Scand 2016;58:75. [Crossref] [PubMed]
- Liu HF, Ren QM, Wang ZB, et al. Comparison of acute phase protein and hemodynamic variables in dogs undergoing video-assisted thoracoscopic vs. open pneumonectomy. Exp Ther Med 2017;13:2391-8. [Crossref] [PubMed]
- Nwogu CE, Yendamuri S, Demmy TL. Does thoracoscopic pneumonectomy for lung cancer affect survival? Ann Thorac Surg 2010;89:S2102-6. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [PubMed]
- Karunanantham J, Fok M, Ali JM, et al. Subxiphoid single incision thoracoscopic surgery approach for thymectomy: a case report. J Vis Surg 2017;3:147. [Crossref] [PubMed]
- Licht PB. Subxiphoid uniportal lobectomy. Eur J Cardiothorac Surg 2016;50:1067. [Crossref] [PubMed]
- Hernandez-Arenas LA, Lin L, Yang Y, et al. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur J Cardiothorac Surg 2016;50:1060-6. [Crossref] [PubMed]
- Nan YY, Chu Y, Wu YC, et al. Subxiphoid video-assisted thoracoscopic surgery versus standard video-assisted thoracoscopic surgery for anatomic pulmonary lobectomy. J Surg Res 2016;200:324-31. [Crossref] [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i54-8. [PubMed]
- Li L, Tian H, Yue W, et al. Subxiphoid vs intercostal single-incision video-assisted thoracoscopic surgery for spontaneous pneumothorax: A randomised controlled trial. Int J Surg 2016;30:99-103. [Crossref] [PubMed]


