A single-institution retrospective analysis of metachronous and synchronous metastatic bronchial neuroendocrine tumors
Introduction
Broncho-pulmonary neuroendocrine neoplasms (bpNENs) originate from a population of neuroendocrine cells present in the bronchoalveolar tissue.
In accordance with the 2015 WHO lung NEN classification (1) bpNENs are sub-divided into well or poorly differentiated forms. Well-differentiated forms, referred to as tumors [neuroendocrine tumors, (NETs)], include low- and intermediate-grade malignancies, i.e., typical (TC) and atypical carcinoid (AC), respectively (2). The poorly differentiated group include large cell neuroendocrine carcinomas (LCNEC) and small cell lung cancer (SCLC) and are referred to as carcinomas [neuroendocrine carcinomas, (NECs)]. The Ki-67 labelling index (Li) is not used to classify bpNENs in accordance with the WHO lung NEN classification, however it can distinguish high-grade bpNENs from low-intermediate grade bp NETs, especially in small biopsies with crushed and/or necrotic tumor cells. Ki-67 remains a helpful parameter, when correlated with mitotic index and necrosis, in therapy choice (1,3).
Although bpNETs are an extremely rare (1–2%) type of bp malignancy, they represent 25–30% of all well differentiated NENs (2). The age-adjusted incidence rate is 1.2/100,000 population/year in European countries, and there has been a gradual annual increase in incidence over the last 30 years of approximately 6.1% (95% CI, 4.7–7.4%) (4).
Some paraneoplastic syndromes are occasionally associated with bpNETs, most commonly the carcinoid syndrome (2–5% of cases) (2,5-7).
About 23% and 28% of patients present with regional and distant metastasis at diagnosis, respectively (8). Metastases may develop metachronously even many years after surgical removal of the primary tumor and regional nodes (9). A relapse rate of 15% in TC has been reported, usually to regional lymph nodes, with a median time to recurrence of 4 years, whereas AC have a relapse rate of 50%, regionally or distantly, with a median time to recurrence of 1.8 years (2).
Typical carcinoids have a relatively good prognosis, with a 5-year survival rate of 87–90%, compared to 44–78% for AC (5).
There is no global consensus on surveillance after complete and radical resection of the primary tumor. Various guidelines recommend different follow-up strategies, in terms of tools and timing (2,5,10).
Due to the rarity of these tumors, identification of independent prognostic factors is lacking and there is no robust evidence regarding behavioral characterization, adjuvant treatment and management of metastatic bpNETs.
Data sharing is therefore extremely important and ideally that every single case should be discussed by a multi-specialist team.
This retrospective analysis aims increase the understanding of metastatic bpNETs, focusing on the pattern of relapse and outcomes of metachronous and synchronous populations.
Methods
Clinical records of patients with a diagnosis of NET, evaluated at the European Institute of Oncology (IEO) between 1997 and 2014, were retrospectively reviewed.
The IEO is a referral centre for NET patients and clinical cases of NET patients are usually discussed by a multidisciplinary NET-dedicated team at weekly meetings. Data for this retrospective analysis were extracted from several sources, including the Thoracic Surgery Division database, the Nuclear Medicine Division database, the Tumor Registry database (data collection on all those consulting at the IEO from 1999 to 2007), outpatient activity Software 2013–2014 (outpatient medical record system), and a database of patients prospectively discussed within the NET tumor board between January 2015 to September 2015).
Cancer staging was reported in accordance with the 7th edition of the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) TNM staging system (11).
“Metachronous” meant that the first diagnosis of the distant metastases was at least 6 months after the date of the primary tumor removal.
Often, due to the poor quality of biological material the pathologist is not always able to differentiate between TC or AC and so NET is used as a common definition or “NOS” (not otherwise specified) bpNET.
We included NOS patients in the analysis as “carcinoid” or “NET” was described in the pathology report.
Primary tumor complete surgery was an anatomic lung resection with adequate lymph node evaluation (at least 6 nodes examined). Three of these nodes had to be mediastinal, including the subcarinal, and 3 from N1 nodes/stations (11,12).
Inclusion criteria:
- histological or cytological diagnosis of bpNETs in accordance with the 2015 WHO lung NEN classification, with distant metastases (metachronous or synchronous);
- sporadic NETs only;
- only patients presenting for the first time at IEO either prior to or concurrent with distant metastases diagnosis (“incident” cases).
Exclusion criteria:
- only locoregional recurrences, secondary tumors within the same lobe, hilum, mediastinal, subcarinal, scalene or supraclavicular lymph nodes;
- non-neuroendocrine malignancies;
- primary extra-pulmonary NET or a histological report of poorly differentiated NEN, LCNEC, SCLC, neuroendocrine cell hyperplasia or tumorlets;
- tumors associated with a multiple endocrine neoplasia (MEN)-1.
Demographics and clinical characteristics, pathology, imaging results as well as surgical and non-surgical treatments were collected retrospectively.
“Ever smokers” included ex-smokers and current smokers.
Follow-up and clinical management was at the discretion of each treating physician.
Recurrence characteristics were evaluated in detail: timing, site, method of detection and Ki-67 index.
To test the prognostic value of different factors, univariate and multivariate survival analyses were performed.
Last updated follow-up data was September 25th, 2015.
A literature search was conducted on PubMed (National Library of Medicine, Bethesda, USA), with a date range from 1970 to 2017 using the Medical Subject (MeSH) Headings “Carcinoid Tumor” linked to “Lung Neoplasms” and the text words ‘pulmonary NETs’, ‘bronchial NETs’, ‘lung NETs’, ‘carcinoid tumors’, ‘bronchial carcinoid tumors’, ‘lung carcinoid tumors’, ‘pulmonary carcinoid tumors’, ‘typical carcinoid’, ‘AC’, ‘NETs’.
MeSH Headings “neoplasm recurrence local” and the truncated word stem “metasta*” were also used.
Abstract communications from the most important conferences on NETs were considerate (ASCO, ESMO, ENETS) from 2013 to the present.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by IEO Ethical Committee (No. R 341/15-IEO 354).
Statistical analysis
Differences between synchronous and metachronous bpNETs in the distribution of categorical variables were tested by the Chi-Square test and the Chi-Square test for trend, as appropriate. Overall survival (OS) was defined as the time interval from date of diagnosis of metastatic bpNET to death from any cause or to last date of follow-up. OS was calculated with the Kaplan-Meier method and compared across different subgroups by means of the Log-rank test. All variables from the univariate analysis were tested in a multivariate Cox regression model, and retained only if they improved the model fit significantly (P≤0.10). Hazard ratios (HR) and 95% confidence intervals (CI) were reported. All analyses were carried out with the Statistical Analysis System (SAS) software (SAS Institute, Cary, NC, USA) and the R (http://cran.r-project.org/) software. All the reported P values were two sided.
Results
Characteristics of the whole study population
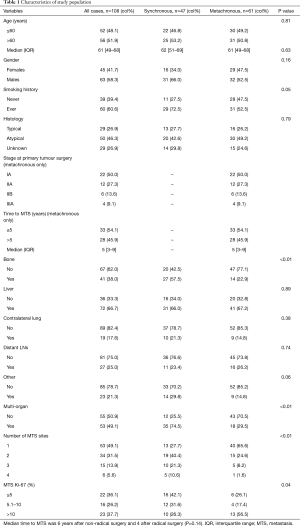
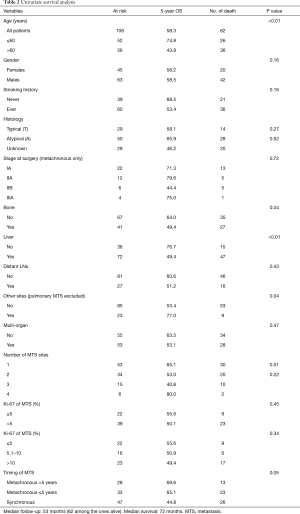
We reviewed 348 clinical records of patients with diagnosis of bpNET. The analysis included 108 patients with metastatic bpNETs, 61 of whom with metachronous and 47 with synchronous metastases. Detailed demographics and clinical characteristics are reported in Table 1. Males (58.3%) and ever smokers (60.6%) were prevalent. Ever smokers had more AC (n=30, 60%) and NOS (n=18, 62%) than TC patients (n=12, 41%). Median age at diagnosis of metastases was 61 years, with an interquartile range (IQR) from 49 to 68 years. Twenty-nine patients (26.9%) had a TC, 50 (46.3%) AC and 29 (26.9%) a not otherwise specified (NOS) bpNET. Functional bpNET were observed in 24 patients (22%), 14 (23%) with metachronous and 10 (21%) synchronous distant metastases; in 79% liver metastases were present. The most frequent paraneoplastic syndrome was carcinoid syndrome (n=22, 92%); ACTH-ectopic syndrome was reported in the remaining cases (n=2, 8%).
Full table
Synchronous metastatic bpNET subgroup characteristics
Synchronous metastatic bpNETs prevailed in males (66%) and in ever smokers (72.5%). Median age at diagnosis of metastases was 62 years. Typical carcinoids and AC were present in 13 (27.7%) and 20 (42.6%) patients, respectively; NOS in 14 (29.8%). The Ki67 LI was available in 38 out of 47 patients (80.8%). It was ≤5% in 16 cases (42%), 6–20% in 19 (50%), and >20% in 3 cases (8%); the median value was 7.5% (range, 0–30%). Patients with synchronous metastases had significantly more metastatic sites, in particular bone (P<0.01), than patients with metachronous disease.
Metachronous metastatic bpNET subgroup characteristics
Median follow-up was 59 [11–215] months, defined as median time between the date of diagnosis of metastatic bpNET to death from any cause or to last date of follow-up.
Gender differences in patients with metachronous metastatic bpNETs were not significant (female/male: 29/32), as well as ever- and never-smokers (52.5% and 47.5%, respectively).
Typical carcinoids and AC were present in 16 (26%) and 30 (49%) patients, respectively; NOS carcinoid in 15 (24.6%).
The 61 metachronous metastatic bpNETs underwent 40 lobectomies (66%), 5 atypical resections, 5 bi-lobectomies and 4 pneumonectomies. In 7 patients the surgical details were not available. Primary tumor complete surgery was performed in 43% cases.
A tumor-node-metastasis (TNM) staging was evaluable in 44 cases (72%) of metachronous disease.
Twenty-nine cases were pT1 and 15 pT2. Thirty-four tumors had no lymph node metastasis (pN0), whereas pathological N1 stage (pN1) was identified in 5 patients and pathological N2 stage (pN2) in 6. Twenty-two cases were Stage IA, 12 Stage IIA, 6 Stage IIB and 4 Stage IIIA (Table 2). Three patients with TC (18.7%) and 7 with AC (23.3%) had lymph node involvement at diagnosis. The Ki67 LI, available in 27 out of 61 patients (44%), was ≤5% in 9 cases (33%), 6–20% in 17 (63%), and >20% in 1 case (4%); median value 11.2% (range, 1–30%).
Full table
Only a few patients received adjuvant treatment: 3 chemotherapy (ChT), 3 radiotherapy (RT), 1 ChT-RT, 1 somatostatin analogue (SSA) and 1 ChT + SSA.
Preoperatively 8 patients had a 18F-deoxyglucose positron emission tomography/computed tomography (18FDG-PET/TC), 6 with a positive (1 TC, 4 AC, 1 NOS) and 2 with a negative (1 TC, 1 AC) result. Finally, 2 patients had a positive (somatostatin receptor scintigraphy) SRS, both AC.
A detailed description of the follow-up procedures was reported for 36% of patients. The follow-up procedures more frequently performed were computed tomography (CT) scan, SRS, liver ultrasound (US), blood chromogranin A (CgA), 18FDG-PET/CT and chest X-ray in selected cases.
Focus on recurrence pattern
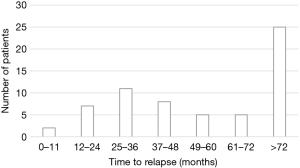
Median time to recurrence (m-TTR) was 5 years with an IQR of 3 to 9 years; surprisingly it was 6 years for non-complete surgical resection (n=35) and 4 years for complete surgical resection (n=26) (P=0.14).
Typical, AC and NOS carcinoids had a m-TTR of 4, 5 [0–18], 3 [1–12] and 9 [3–31] years, respectively.
Time to recurrence distribution, shown in Figure 1, indicated two relapse peaks after primary resection, the former between 25 and 36 months and the latter beyond 72 months.
Liver (67%), extra-regional lymph nodes (26%), bone (23%) and lung (15%) were the most common sites of recurrence, along with subcutis (6%).
The most common methods of first recurrence detection were CT scan (19 cases), liver US in 5 patients, SRS in 3, CgA levels, 18FDG-PET/CT and magnetic resonance imaging (MRI) in 2, 68Ga-DOTA-peptide PET/CT and chest X-ray in 1.
The Ki67 LI of the site of recurrence was available in 23 out of 61 patients (38%). It was ≤5% in 6 cases (26%), 6–20% in 15 (65%) and >20% in 2 cases (9%); the median value was 14% (range, 0–60%).
Survival analysis for the whole population
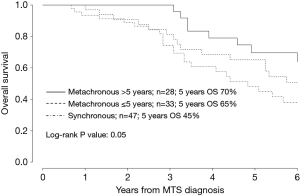
Median follow-up was 53 [9–215] months, defined as the median time between the date of diagnosis of metastatic bpNET to death from any cause or to last date of follow-up. Number of deaths from any cause was 62/108 and 5-year OS was 58.3% for the whole population. The 5-year OS rate was 69.6% in metachronous disease with a TTR >5 years, 65% in metachronous disease with TTR ≤5 years and 44.8% in synchronous disease (Figure 2).
Median OS from the date of diagnosis of distant metastasis was 72 months for the whole population. Median OS for metachronous disease with TTR >5 years after primary diagnosis was 78 months, 73 months for metachronous disease with TTR <5 years and 53 months for synchronous disease.
Univariate survival analysis
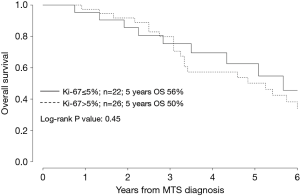
Age >60 years (P<0.01), bone metastasis (P=0.04), liver metastasis (P<0.01), Ki-67 as a continuous variable (P≤0.10) and shorter TTR (P=0.05) significantly correlated with poorer prognosis (Table 2, Figures 2,3).
Tumor stage at surgery was not associated with survival, for metachronous disease; particularly 5-year OS was 71.3% for Stage IA, 79.6% for Stage IIA, 44.4% for Stage IIB and 75% for Stage IIIA.
Multivariate survival analysis
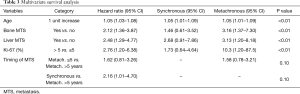
Age (HR of 1.05), bone metastasis (HR of 2.12), liver metastasis (HR of 2.48), Ki-67 as a continuous variable (HR of 1.07) significantly correlated with prognosis (Table 3, Figure 3).
Full table
Moreover, there was a significant positive correlation between TTR and survival (Figure 2).
Therapies of metastatic bpNETs
As shown in Table 4 the most common first-line therapy performed was SSA in 43 (40%) patients (in 13 cases associated with other therapies), followed by peptide receptor radionuclide therapy (PRRT) in 20 (19%), surgery in 20 (19%) and platinum-etoposide ChT in 13 (12%). Liver resection was performed in 5 cases (all metachronous) and lung (primary +/− metastasis) surgical resection in 12 cases. Somatostatin analogues were administered as a first-line in 90% of TC and in 38% of AC. The subgroup analysis of patients with ChT and surgery as first-line therapy showed that diagnosis of metastatic disease was before 2010 in 74% and 70% cases respectively.

Full table
Discussion
This report represents one of the largest series of patients with bpNET with distant metastases managed in a single institution, with a long-term follow-up (median follow-up was 59 months), similar to previously published reports (7,13-16).
The results of this analysis are limited due to the long time interval for data collection (1997 to 2014), variety of therapeutical and follow-up management and lack of retrospective centralized pathological review.
The population characteristics of metastatic bpNETs had a median age of 61 years, similar to previous reports (6,7,17-21). The prevalence of ever smokers (60%) was discrepant with previous studies, which reported lower prevalences (32.5–53%) (6,7,13,21-23). in this study AC patients (60%) were more frequently ever smokers than TC patients (41%), in line with other published studies (6,7,24).
Comparison between synchronous and metachronous metastatic bpNETs indicated a prevalence of males and ever smokers in the synchronous population, similar median age at diagnosis of metastases and similar distribution between TC, AC and NOS.
Patients with synchronous disease had significantly more metastatic sites, in particular bone, and lower Ki-67 LI than patients with metachronous metastases.
Among metachronous bpNET patients, the most frequent primary surgical approach was lobectomy, as reported in other studies (15,16,21,25), and complete resection was described in only 43% of cases. Surgical procedures were performed in several Centres nationally, only a minority of the cases, all complete), at IEO.
Notably description of completeness of surgical resection is missing in several studies on bpNETs (26-28).
There is no consensus on adjuvant therapy for bpNETs. More in general it is not recommended, even though some guidelines suggest that either ChT (more often platinum-etoposide) and/or RT should be discussed in intermediate grade (atypical, high Ki-67) and intermediate stage (pN+) on an individual patient basis (2,29).
The 5 years median TTR observed in our study was longer compared with that reported in other studies (20,21,30,31).
Surprisingly there was a shorter TTR after complete primary tumor resection compared with incomplete (4 and 6 years, respectively). Several reasons may explain this including different biology of the disease and different follow-up. Therefore, we think that nodal metastases should be confirmed as one of the most important prognostic factors (9,13,31-33).
The shorter m-TTR observed in patients with AC compared with TC (3 and 4.5 years, respectively) and recurrence time distribution (around 50% of recurrences happened after 5 years) was in line with other reports (6,21,23,33,34).
Of note, distant metastases showed higher KI67 LI compared with primary sites.
Unfortunately a detailed description of the follow-up procedures was reported in only 36% of patients, because of the long period of the cases included and lack of completeness of medical records.
The most common method of follow-up and detection of first recurrence was CT scan, as described in other studies (14,15,21,25,34-36).
Our personal recommendation for surveillance is Chest-Abdomen CT every 6 months for the first 3 years. Thereafter patients should be followed annually for up to 15 years. Biochemical markers, such as CgA, should be determined every 6 months (in cases with elevated values at baseline).
Median OS for the whole population is similar to the 6.6 years of mOS reported in a recent large series of metastatic bpNETs (7).
No significant prognostic difference were observed between TC and AC based on different stages at surgery (unlike previous studies) (7,9,14,23,32,34,37) and between synchronous and metachronous, even though patients with synchronous metastases tended to have a shorter survival than patients with metachronous metastases.
Age, bone metastasis, liver metastasis and Ki-67 as a continuous variable significantly correlated with prognosis at multivariate analysis. Moreover, there was a significant positive correlation between TTR and survival.
This could have conditioned some results, such as the surprising statistical correlation between pTNM staging, complete surgery and histotype and clinical outcome.
In Table 4 we describe the most frequent first and subsequent lines of therapy.
Although we have these data, we avoided a retrospective and misleading analysis of treatment efficacy because of long period of time covering the selected cases and the variation in therapeutical approaches.
In our study SSA was the most frequent first-line therapy (40% patients). Although there is no absolute evidence of efficacy of SSA as anti-tumor therapy in bpNET, they are usually proposed after extrapolating data from the GEP tract evidence.
Studies of therapies in the advanced or metastatic lung carcinoid setting have been limited to retrospective analyses and some small prospective studies (6,38-40). Recent articles reviewed the current standard of care and recent advances in therapy for bpNETs (41-44).
In conclusion, this is one of the largest single-centre series of metastatic bpNETs. Although many questions still remain open: difference between typical and atypical behavior, role of functional imaging in diagnostic workup, tools and timing of follow-up after a complete resection of the primary tumor and identification of independent prognostic factors (i.e., KI-67), the results of our retrospective analysis represent a basis for designing prospective clinical trials, in adjuvant and metastatic settings, for homogeneous bpNETs populations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by IEO Ethical Committee (No. R 341/15-IEO 354).
References
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. ENETS consensus conference participants. Ann Oncol 2015;00:1-17.
- Volante M, Gatti G, Papotti M. Classification of lung neuroendocrine tumors: lights and shadows. Endocrine 2015;50:315-9. [Crossref] [PubMed]
- Boyar Cetinkaya R, Aagnes B, Thiis-Evensen E, et al. Trends in Incidence of Neuroendocrine Neoplasms in Norway: A Report of 16,075 Cases from 1993 through 2010. Neuroendocrinology 2017;104:1-10. [Crossref] [PubMed]
- Öberg K, Hellman P, Ferolla P, et al. Neuroendocrine bronchial and thymic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up; ESMO Guidelines Working Group. Ann Oncol 2012;23 Suppl 7:vii120-3. [PubMed]
- Chong CR, Wirthb LJ, Nishino M, et al. Chemotherapy and irradiation for locally advanced and metastatic pulmonary carcinoid tumors. Lung Cancer 2014;86:241-6. [Crossref] [PubMed]
- Forde PM, Hooker CM, Boikos SA. Systemic Therapy, Clinical Outcomes, and Overall Survival in Locally Advanced or Metastatic Pulmonary Carcinoid, A Brief Report. J Thorac Oncol 2014;9:414-8. [Crossref] [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Filosso PL, Ferolla P, Guerrera F, et al. Multidisciplinary management of advanced lung neuroendocrine tumors. J Thorac Dis 2015;7:S163-71. [PubMed]
- Phan AT, Oberg K, Choi J, et al. NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the thorax (includes lung and thymus). Pancreas 2010;39:784-98. [Crossref] [PubMed]
- TNM Classification of Malignant Tumours. Seventh edition 2009. Available online: https://www.uicc.org/sites/main/files/private/TNM_Classification_of_Malignant_Tumours_Website_15%20MAy2011.pdf
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Cardillo G, Sera F, Di Martino M, et al. Bronchial Carcinoid Tumors: Nodal Status and Long-Term Survival After Resection. Ann Thorac Surg 2004;77:1781-5. [Crossref] [PubMed]
- Maurizi G, Ibrahim M, Andreetti C, et al. Long-term results after resection of bronchial carcinoid tumour: evaluation of survival and prognostic factors. Interact Cardiovasc Thorac Surg 2014;19:239-44. [Crossref] [PubMed]
- Srirajaskanthana R, Toumpanakisa C, Karpathakis A. Surgical management and palliative treatment in bronchial neuroendocrine tumours: A clinical study of 45 patients. Lung Cancer 2009;65:68-73. [Crossref] [PubMed]
- Lequaglie C, Patriarca C, Cataldo I, et al. Prognosis of resected well-differentiated neuroendocrine carcinoma of the lung. Chest 1991;100:1053-6. [Crossref] [PubMed]
- Sullivan I, Baudin E, Guigay J. Tumor control of advanced pulmonary carcinoid tumors with somatostatin analogs: Experience at Gustave Roussy. 2015 ESMO Annual Meeting.
- Diamantopoulos LN, Demetriou GA, Laskaratos FM, et al. Bronchial Neuroendocrine Tumours with Advanced Disease: A Misleading Biology. 2017 ENETS Annual Meeting.
- Mandair D, Diamantopoulos LN, Demetriou, et al. Typical bronchial NETs as a misleading biology. 2017 ASCO Annual Meeting.
- Walter T, Planchard D, Bouledrak K, et al. Characterization and prognosis of patients with metastatic lung carcinoid tumors. 2015 ENETS Annual Meeting.
- Lou F, Sarkaria I, Pietanza C, et al. Recurrence of pulmonary carcinoid tumors after resection: implications for postoperative surveillance. Ann Thorac Surg 2013;96:1156-62. [Crossref] [PubMed]
- Hassan MM, Phan A, Li D, et al. Risk factors associated with neuroendocrine tumors: a U.S.-based case-control study. Int J Cancer 2008;123:867-73. [Crossref] [PubMed]
- Lee PC, Osakwe NC, Narula N, et al. Predictors of Disease-free Survival and Recurrence in Patients with Resected Bronchial Carcinoid Tumors. Thorac Cardiovasc Surg 2016;64:159-65. [PubMed]
- Faggiano A, Ferolla P, Grimaldi F, et al. Natural history of gastro-entero-pancreatic and thoracic neuroendocrine tumors. Data from a large prospective and retrospective Italian Epidemiological study: the NET MANAGEMENT study. J Endocrinol Invest 2012;35:817-23. [PubMed]
- Davini F, Gonfiotti A, Comin C, et al. Typical and atypical carcinoid tumours: 20-year experience with 89 patients. J Cardiovasc Surg (Torino) 2009;50:807-11. [PubMed]
- Warren WH, Gould VE. Long-term follow-up of classical bronchial carcinoid tumors. Clinicopathologic observations. Scand J Thorac Cardiovasc Surg 1990;24:125-30. [Crossref] [PubMed]
- Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001;119:1647-51. [Crossref] [PubMed]
- Asamura H, Kameya T, Matsuno Y. Neuroendocrine Neoplasms of the Lung: A Prognostic Spectrum. J Clin Oncol 2006;24:70-6. [Crossref] [PubMed]
- NCCN Guidelines Version 3.2017 Neuroendocrine Tumors of the Gastrointestinal Tract, Lung, and Thymus (Carcinoid Tumors).
- Filosso PL, Rena O, Donati G, et al. Bronchial carcinoid tumors: surgical management and long-term outcome. J Thorac Cardiovasc Surg 2002;123:303-9. [Crossref] [PubMed]
- Cañizares MA, Matilla JM, Cueto A, et al. Atypical carcinoid tumours of the lung: prognostic factors and patterns of recurrence. Thorax 2014;69:648-53. [Crossref] [PubMed]
- Filosso PL, Ruffini E, Di Gangi S, et al. Prognostic factors in neuroendocrine tumours of the lung: a single-centre experience. Eur J Cardiothorac Surg 2014.521-6. [Crossref] [PubMed]
- Cusumano G, Fournel L, Strano S, et al. Surgical Resection for Pulmonary Carcinoid: Long-Term Results of Multicentric Study-The Importance of Pathological N Status, More Than We Thought. Lung 2017;195:789-98. [Crossref] [PubMed]
- Feczko AF. Patterns of Recurrence and Methods of Surveillance in Low- and Intermediated-Grade Neuroendocrine Tumors. 2018 Society of Thoracic Surgeons Annual Meeting.
- Zanata I. Lung Carcinoid: Role of NSE and Imaging Techniques in Long-Term Follow-Up of Malignancy Recurrence. 2017 ENETS Annual Meeting.
- Pusceddu S, Catena L, Valente M. Long-term follow up of patients affected by pulmonary carcinoid at the Istituto Nazionale Tumori of Milan: a retrospective analysis. J Thorac Dis 2010;2:16-20. [PubMed]
- Travis WD, Giroux DJ, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the inclusion of broncho-pulmonary carcinoid tumors in the forthcoming (seventh) edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2008;3:1213-23. [Crossref] [PubMed]
- Walter T, Planchard D, Bouledrak K. Evaluation of the combination of oxaliplatin and 5-fluorouracil or gemcitabine in patients with sporadic metastatic pulmonary carcinoid tumors. Lung Cancer 2016;96:68-73. [Crossref] [PubMed]
- Crona J, Fanola I, Lindholm DP, et al. Effect of temozolomide in patients with metastatic bronchial carcinoids. Neuroendocrinology 2013;98:151-5. [Crossref] [PubMed]
- Mariniello A, Bodei L, Tinelli C, et al. Long-term results of PRRT in advanced bronchopulmonary carcinoid. Eur J Nucl Med Mol Imaging 2016;43:441-52. [Crossref] [PubMed]
- Fazio N, Ungaro A, Spada F, et al. The role of multimodal treatment in patients with advanced lung neuroendocrine tumors. J Thorac Dis 2017;9:S1501-10. [Crossref] [PubMed]
- Wolin EM. Advances in the Diagnosis and Management of Well-Differentiated and Intermediate-Differentiated Neuroendocrine Tumors of the Lung. Chest 2017;151:1141-6. [Crossref] [PubMed]
- Hilal T. Current understanding and approach to well differentiated lung neuroendocrine tumors: an update on classification and management. Ther Adv Med Oncol 2017;9:189-99. [Crossref] [PubMed]
- Bouledrak K, Walter T, Souquet PJ, et al. Metastatic bronchial carcinoid tumors. Rev Pneumol Clin 2016;72:41-8. [Crossref] [PubMed]