Outcomes of surgical aortic valve replacement for mixed aortic valve disease
Introduction
Mixed aortic valve disease (MAVD) is associated with a high rate of disease progression with 42% and 30% freedom from the composite end points of aortic valve replacement (AVR), NYHA III/IV symptoms, and death at 3 and 5 years respectively (1,2). Indeed, the natural history outcomes of mixed moderate aortic valve (AV) disease were similar to isolated severe AV disease in terms of progression to AVR, NYHA III/IV symptoms, and cardiac death. This moderate MAVD therefore has a poorer prognosis than isolated moderate aortic stenosis (AS) or aortic insufficiency (AI) (1). Zilbersac et al. demonstrated a similarly high event rate with event free survival (AVR and cardiac death) as low as 19% at 6 years for MAVD where both lesions were moderate or greater (3).
Approximately 50% of asymptomatic patients with severe MAVD will require AVR within 1 year (4). As suggested by Parker et al., this is not surprising due to the dual volume and pressure strain on the heart (5). Although we are gaining a better understanding of the aggressive course of MAVD, there are currently no evidence-based recommendations to guide timing of surgical AVR in this population (3,6). In general, surgical intervention is often guided by established recommendations for the predominate lesion (3,6,7). Egbe et al. showed that in MAVD with moderate or severe AS and AI, a greater degree of pre-operative left ventricular hypertrophy (LVH) is associated with more post-operative left ventricular (LV) dysfunction and cardiac adverse events (8,9). This study seeks to further define the long term clinical and echocardiographic outcomes for AVR in MAVD versus more isolated AV lesions.
Methods
Patients
This study was approved by the University of Wisconsin-Madison Institutional Review Board (No. 2015-1050). A waiver of the need to obtain consent from patients was approved. We conducted a retrospective review of 1,011 consecutive patients who underwent AVR between January 2000 to March 2016. We excluded patients who underwent concomitant operations except for coronary artery bypass grafting (CABG) and/or limited ascending aortic replacement. We excluded aortic surgeries involving the root, or aortic arch, as well as procedures involving hypothermic circulatory arrest.
We identified patients who underwent AVR for predominantly AS or AI where associated stenosis or insufficiency are not greater in severity than the designated predominant lesion. The predominant AS population with severe or moderate stenosis was stratified into group 1 (n=660) with concomitant AI that was mild or less, and group 2 (n=197) with accompanying moderate or severe AI. Similarly, the predominant AI group with severe to moderate AI was stratified using the same schema into group 3 (n=143) with concomitant AS that was mild or less, and group 4 (n=53) with accompanying moderate or severe AS.
Follow up
Survival data was available for all 1,100 patients with AVR. Mid-term survival data was obtained through detailed clinical follow-up. For the predominantly AS group maximum imaging follow-up was 16.5 years with a total follow up of 3,384.3 patient years and a median follow up of 3.1 (IQR =5.4) years. The predominant AI group maximum imaging follow-up was 16.8 years with a total follow up of 1,025.1 patient years and a median follow up of 4.4 (IQR =7.0) years.
Statistical methods
A Pearson chi square test or Fisher’s exact test was used to analyze categorical variables. Independent and paired Student’s t-test was used to compare continuous variables. Kaplan-Meier Survival analysis with Mantel-Cox statistics was used to analyze survival data. Life expectancy was calculated using life table analysis. The year of operation is compared with the median test.
A logistical regression of covariates was used to produce a propensity score for patients with predominantly moderate or severe AS where associated insufficiency was either mild or less (group 1), or moderate and severe (group 2). Similarly, propensity score calculation was performed for the predominant AI population for groups 3 and 4. Then 1:1 greedy matching algorithm was performed where the matched variables included age, sex, preoperative creatinine, left ventricular ejection fraction (LVEF), hypertension, cerebrovascular disease, peripheral vascular disease, lung disease, liver disease, diabetes, hyperlipidemia, history of endocarditis, and cancer within 5 years of surgery. A Cox proportional hazards model using univariate as well as stepwise forward and reverse multivariable analysis was performed to determine the predictors of mortality. Statistics were performed using Statistical Package for the Social Sciences software (SPSS Inc., Chicago, IL, USA).
Results
Patient demographics, operative parameters and outcomes
Compared with group 2, the predominant AS group 1 population was older (70.0±10.7 vs. 67.9±13.4 yrs, P=0.041), had higher LVEF (59.6±12.6 vs. 56.7±13.5 yrs, P=0.008), more diabetes (35.0% vs. 23.9%, P=0.003), more hyperlipidemia (75.8% vs. 65.0%, P=0.003), but a lower incidence of endocarditis history (0.6% vs. 2.5%, P=0.020). There were 195 patients in each group following propensity matching with comparable demographics and comorbidities (Table 1). There were no differences in concomitant operations performed for the 2 groups (Table 2). However, group 2 has a greater duration of cardiopulmonary bypass (CPB) (150.5±55.2 vs. 163.5±57.5, P=0.025) and aortic cross-clamp time (101.7±37.9 vs. 110.3±41.0 min, P=0.042). There were no differences in postoperative complications including reoperation for bleeding, neurological events, pneumonia, prolonged ventilation, gastrointestinal complication, acute renal failure, permanent pacemaker implant, postoperative atrial fibrillation, or postoperative hospital stay (P>0.05).
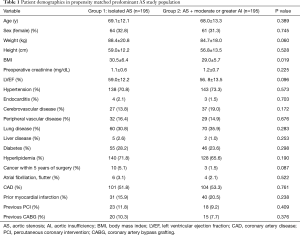
Full table

Full table
Conversely, compared with group 4, the predominant AI group 3 population was younger (56.4±15.6 vs. 61.7±16.3 yrs, P=0.037), and a higher incidence of endocarditis history (32.2% vs. 3.8%, P<0.001). Following propensity matching, there were 53 patients in each group with similar demographics and comorbidities (Table 3). There were no differences in concomitant operations performed for the 2 groups (Table 4). Group 4 had a greater duration of CPB (149.3±52.0 vs. 191.9±81.7, P=0.002) and aortic cross-clamp time (101.9±34.3 vs. 129.1±53.2 min, P=0.006). There were no differences in postoperative complications including reoperation for bleeding, neurological events, pneumonia, prolonged ventilation, gastrointestinal complication, acute renal failure, permanent pacemaker implant, postoperative atrial fibrillation, or postoperative hospital stay (P>0.05).
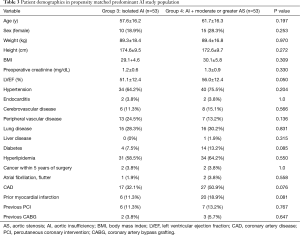
Full table

Full table
Echocardiographic outcomes in MAVD
In the predominant AS group, the median echocardiographic follow up for groups 1 and 2 respectively were 1.926 yrs (IQR =4.72) and 1.770 yrs (IQR =4.65) (P=0.561). There were no differences in AV mean gradient, AV peak velocity, aortic valve area (AVA), or indexed AVA between the two groups (Table 5, P>0.05). Other echocardiographic findings are summarized in Table 6. Both groups had a reduction in left ventricular end diastolic diameter (LVEDD) (group 1: 48.6±7.2 vs. 46.6±7.3 mm, P<0.001 and group 2: 50.9±8.4 vs. 46.9±7.4 mm, P<0.001) after AVR although initial preoperative LVEDD was larger in group 2 (P=0.022). No difference in postoperative LVEDD was seen after AVR (P=0.756). After AVR, both group 1 and 2 had a decrease in LV posterior wall (PW) thickness (group 1, P<0.001; group 2, P=0.002), decrease in interventricular septal (IVS) thickness (group 1, P=0.002; group 2, P=0.005), and reduction in mitral regurgitation (group 1, P<0.001; group 2, P<0.001). However, right ventricular (RV) function worsened over time in both group 1 (P<0.001) and 2 (P=0.030). Preoperative magnetic resonance (MR) was more severe in group 2 (P<0.001) but this difference resolved following AVR (P=0.750). LVEF did not improve significantly following AVR for Group 1 (P=0.128), but improved for group 2 with more severe associated AI (P=0.033). Group 2 had a lower preoperative LVEF (P=0.043), but no difference in postoperative LVEF was seen after AVR (P=0.469).

Full table
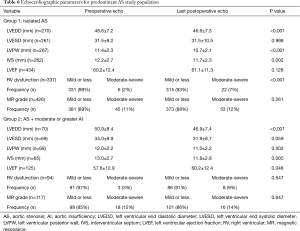
Full table
The median echocardiographic follow up the predominant AI groups 3 and 4 respectively were 3.6 yrs (IQR =6.61) and 1.2 yrs (IQR =4.0) (P=0.004). As expected, compared with group 3, group 4 had more severe AS with higher AV mean gradient and peak AV velocity as well as lower AVA and indexed AVA (Table 5, P<0.001). Other echocardiographic findings are summarized in Table 7. Reduced LV size after AVR was demonstrated in both group 3 (LVEDD, P<0.001; LVESD P=0.001), and 4 (LVEDD, P=0.001; LVESD, P=0.083). There was no difference between group 3 or group 4 in pre (P=0.111) or postoperative (P=0.238) LVEDD. Compared with group 3 however, group 4 had a thicker preoperative PW (P=0.041) and preoperative IVS (P=0.003), but no difference was seen in PW (P=0.719) or IVS (P=0.682) thickness after AVR. In both groups, there was a reduction in mitral regurgitation (group 3, P<0.001; group 4, P=0.002). After AVR, there was no change in RV function in group 3 (P=0.697), but there was a trend toward worsening RV function (P=0.084) in group 4 with a greater degree of associated AS. Pre and postoperative LVEF did not change significantly for either group 3 (0.571), or group 4 (P=0.741).
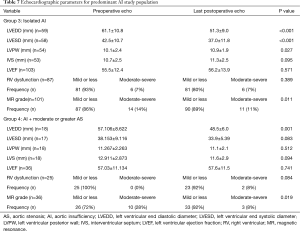
Full table
Patient survival
For the predominant AS group, there were no differences in survival respectively in the unadjusted (P=0.389), and the propensity matched population (P=0.191, Figure 1A). For the propensity matched population, the 3 months, 6 months, 1 year, 2 years, 5 years, and 10 years’ survival respectively were 96.2%, 95.5%, 91.5%, 84.3%, 72.9% and 55.1% for group 1, and 99.0%, 94.7%, 92.7%, 77.6%, and 58.9% for group 2. Cox proportional hazards regression revealed increased hazard ratios (HR) associated with increasing age [HR 1.38 (1.022–1.054), P<0.001], higher preoperative creatinine [HR 1.339 (1.225–1.463), P<0.001], cerebrovascular disease [HR 1.912 (1.384–2.640), P<0.001], and increasing NYHA class [HR 1.427 (1.203–1.693), P<0.001]. Neither group 1 vs. 2 (P=0.429), nor operating surgeon (P=0.103) was a predictor of mortality. The 30-day operative mortality in this group was 2.0%.
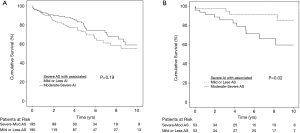
In the predominant AI group, group 4 with more severe associated AS experienced worse survival for both the unadjusted (P=0.030) and propensity matched population (P=0.024, Figure 1B). For the propensity matched population, the 3 months, 6 months, 1 year, 2 years, 5 years and 10 years’ survival respectively were 98.0%, 98.0%, 98.0%, 98.0%, 91.4% and 85.7% for group 3, and 96.2%, 94.0%, 79.4% and 59.7% for group 4. Cox proportional hazards regressive revealed increased HR associated with increasing age [HR 1.36 (1.008–1.064), P=0.010], higher preoperative creatinine [HR 1.356 (1.171–1.571), P<0.001], group 4 has higher hazard vs. group 3 [HR 2.039 (0.939–4.428), P=0.072], Peripheral vascular disease [HR 2.467 (1.079–5.638), P=0.032], previous percutaneous coronary intervention (PCI) [HR 3.294 (1.195–9.081), P=0.021] and increasing NYHA class [HR 1.860 (1.184–2.921), P=0.007]. Neither history of endocarditis (P=0.563), nor operating surgeon (P=0.974) was a predictor of survival. The 30-days operative mortality in this group was 2.2%.
Discussion
There is a paucity of published data on the relative outcomes of surgical AVR in predominant AS and AI lesions versus those with concomitant moderate or greater corresponding stenotic or regurgitant AV pathology. This study revealed that associated AI in the predominant AS population was less detrimental to long term survival than concomitant AS in the predominant AI population. Patients undergoing AVR for predominant AI with associated moderate or severe AS should be closely monitored for symptom progression, decline in LVEF, and increase in LV dimensions. A low threshold for surgical intervention may be reasonable for this population of patients.
Patients with AV stenosis and/or aortic regurgitation are subjected to increased pressure and/or volume overload of the left ventricle, leading to concentric or eccentric LVH (10,11). In the AS group, group 1 had a smaller LVEDD than group 2 with more severe associated AI. This is consistent with the eccentric hypertrophy expected in group 2 as opposed to concentric hypertrophy in group 1. However, reverse remodeling with decreased IVS and PW thickness after AVR resulted in no difference in LV dimensions on follow up echo. These findings are consistent with prior studies examining AS with concomitant AI where appropriate preoperative LV adaptation that preserves systolic function was associated with preserved postoperative LV function after surgical AVR regardless of the coexisting degree of preoperative AI (12). It is important to point out that this is distinct from acute regurgitant volume from a paravalvular leak following transcatheter AVR which predicts a worse outcome in early and intermediate follow-up (13).
Indeed, LVEF improved in those with worse AI (group 2) to match those with less AI (group 1) after AVR. Furthermore, MR improved to comparable levels in both group 1 and 2 despite group 2 having worse MR initially. Similar to Catovic et al. and others, we did not find a difference in survival following AVR between isolated AS and AS associated with significant AI (12,14). Previous authors have demonstrated that AVR for isolated AS or MAVD had 5 and 10 years’ postoperative survivals of 85% and 68% respectively (12). This is comparable to our 5 and 10 years’ survival in a similar population of 77% and 52.2% respectively. As expected, associated AI was not predictive of survival in our Cox multivariable analysis.
Gilbert et al. reported that predominant AI with associated AS in MAVD has different effects on the LV where patients are more likely to have LVH, diastolic dysfunction, and less LV dilatation compared with isolated AI (15). When compared with group 3 on preop echo, we found that group 4 had greater LVH but there was no difference in the degree of LV dilatation. In our study, while the LV dimensions did decrease after AVR, there was no change in pre vs. postop PW and IVS. thickness in group 4. Ali et al. demonstrated that incomplete regression of LVH after AVR is associated with decreased survival (16), and LVH is a risk factor for cardiac morbidity and mortality (17). However, we recognize that in most patients with AI, LVEDD and volumes became near normal within 2 weeks after AVR, where as a significant regression of LVH took at least 6 months (18). Our follow-up period of 1.2 years in this group should have been sufficient to detect decreases in wall thickness.
Accordingly, the AI group with more severe AS has a lower 10 years’ survival of 59.7% compared with group 3 at 85.7% with less severe AS. This is consistent with findings by Rashedi et al. who found that in the natural history of MAVD, the severity of AS had the strongest correlation with progression to death, symptoms, and AVR (6). We demonstrate that this trend was also applicable for survival post-AVR in MAVD for predominant AI lesions. Rashedi et al. also found that MAVD was a more aggressive disease process with more rapid progression to LV systolic dysfunction at 4.3%/yr (6) in contrast to the 1.2%/yr in predominant AI patients (19). Similar to pre-surgical natural history findings by Egbe et al. (4), we also found that LV dimension was not a predictor of survival in MAVD after AVR. Une et al., also reported that despite improvements in LVEF the clinical effects of LVEF recovery was poorer in patients with AI compared with AS (20). Our finding that RV function tend to decline following AVR in patients with worse AS in the predominant AI group may also explain a lower survival in group 4 compared to group 3.
This study has limitations due to its retrospective nature in a single institution with inherent limitations and biases. While propensity matching adjusted for certain differences in patient demographics, and comorbidities, it is possible that other unidentified characteristics had influenced our results. Since our survival data was obtained from hospital records, we may not have captured all mortality events. A decrease in study population following propensity matching may also limit our ability to detect smaller differences in clinical outcomes.
In conclusion, our study confirms that in the predominant AS population, similar perioperative and survival outcomes can be expected after AVR between patients with isolated AS, and AS associated with significant AI. However, in the predominant AI population, survival outcomes after AVR were poorer in AI associated with significant AS than in isolated AI. These data may influence the recommendation on echocardiographic surveillance frequency and indications for surgery in this MAVD population.
Acknowledgements
We acknowledge the administrative support of Entela Bua Lushaj in the conduct of this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the University of Wisconsin-Madison Institutional Review Board (No. 2015-1050). A waiver of the need to obtain consent from patients was approved.
References
- Egbe AC, Luis SA, Padang R, et al. Outcomes in Moderate Mixed Aortic Valve Disease: Is it Time for a Paradigm Shift? J Am Coll Cardiol 2016;67:2321-9. [Crossref] [PubMed]
- Popescu AC, Antonini-Canterin F, Enache R, et al. Impact of associated significant aortic regurgitation on left ventricular remodeling and hemodynamic impairment in severe aortic valve stenosis. Cardiology 2013;124:174-81. [Crossref] [PubMed]
- Zilberszac R, Gabriel H, Schemper M, et al. Outcome of combined stenotic and regurgitant aortic valve disease. J Am Coll Cardiol 2013;61:1489-95. [Crossref] [PubMed]
- Egbe AC, Poterucha JT, Warnes CA. Mixed aortic valve disease: midterm outcome and predictors of adverse events. Eur Heart J 2016;37:2671-8. [Crossref] [PubMed]
- Parker MW, Aurigemma GP. The Simple Arithmetic of Mixed Aortic Valve Disease: LVH + Volume Load = Trouble. J Am Coll Cardiol 2016;67:2330-3. [Crossref] [PubMed]
- Rashedi N, Popovic ZB, Stewart WJ, et al. Outcomes of asymptomatic adults with combined aortic stenosis and regurgitation. J Am Soc Echocardiogr 2014;27:829-37. [Crossref] [PubMed]
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [Crossref] [PubMed]
- Egbe AC, Warnes CA. Cardiovascular Adverse Events After Aortic Valve Replacement in Mixed Aortic Valve Disease: Beyond Ejection Fraction. J Am Coll Cardiol 2016;68:2591-3. [Crossref] [PubMed]
- Egbe AC, Warnes CA. Predictor of left ventricular dysfunction after aortic valve replacement in mixed aortic valve disease. Int J Cardiol 2017;228:511-7. [Crossref] [PubMed]
- Monrad ES, Hess OM, Murakami T, et al. Time course of regression of left ventricular hypertrophy after aortic valve replacement. Circulation 1988;77:1345-55. [Crossref] [PubMed]
- Orsinelli DA, Aurigemma GP, Battista S, et al. Left ventricular hypertrophy and mortality after aortic valve replacement for aortic stenosis. A high risk subgroup identified by preoperative relative wall thickness. J Am Coll Cardiol 1993;22:1679-83. [Crossref] [PubMed]
- Lund O. Preoperative risk evaluation and stratification of long-term survival after valve replacement for aortic stenosis. Reasons for earlier operative intervention. Circulation 1990;82:124-39. [Crossref] [PubMed]
- Lerakis S, Hayek SS, Douglas PS. Paravalvular aortic leak after transcatheter aortic valve replacement: current knowledge. Circulation 2013;127:397-407. [Crossref] [PubMed]
- Catovic S, Popovic ZB, Tasic N, et al. Impact of concomitant aortic regurgitation on long-term outcome after surgical aortic valve replacement in patients with severe aortic stenosis. J Cardiothorac Surg 2011;6:51. [Crossref] [PubMed]
- Gilbert JC, Glantz SA. Determinants of left ventricular filling and of the diastolic pressure-volume relation. Circ Res 1989;64:827-52. [Crossref] [PubMed]
- Ali A, Patel A, Ali Z, et al. Enhanced left ventricular mass regression after aortic valve replacement in patients with aortic stenosis is associated with improved long-term survival. J Thorac Cardiovasc Surg 2011;142:285-91. [Crossref] [PubMed]
- Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322:1561-6. [Crossref] [PubMed]
- Carroll JD, Gaasch WH, Zile MR, et al. Serial changes in left ventricular function after correction of chronic aortic regurgitation. Dependence on early changes in preload and subsequent regression of hypertrophy. Am J Cardiol 1983;51:476-82. [Crossref] [PubMed]
- Bonow RO, Rosing DR, McIntosh CL, et al. The natural history of asymptomatic patients with aortic regurgitation and normal left ventricular function. Circulation 1983;68:509-17. [Crossref] [PubMed]
- Une D, Mesana L, Chan V, et al. Clinical Impact of Changes in Left Ventricular Function After Aortic Valve Replacement: Analysis From 3112 Patients. Circulation 2015;132:741-7. [Crossref] [PubMed]


