Extracranial metastatic burden in extensive-stage small cell lung cancer: implications for prophylactic cranial irradiation
Introduction
Small cell lung cancer (SCLC) is an aggressive malignancy characterized by its distinct morphologic features and strong correlation with a history of smoking. SCLC accounts for 10% of all lung cancer cases (1). It has been determined that combined radiation and chemotherapy is the most effective standard treatment for limited-stage small cell lung cancer (LS-SCLC) (2). For extensive-stage small cell lung cancer (ES-SCLC), cytotoxic chemotherapy with a platinum-based compound and etoposide remains the standard of care. Recently a phase III randomized controlled trial established a survival benefit for patients with the addition of consolidative thoracic radiotherapy following response to chemotherapy (3).
Patients with ES-SCLC are predisposed toward developing brain metastases. While prophylactic cranial irradiation (PCI) has been established as standard treatment for patients with LS-SCLC who have a complete response to chemotherapy (4), there is significant controversy regarding the use of PCI for patients with ES-SCLC. Slotman et al. published a phase III randomized controlled study which established a survival benefit with PCI with the routine use of this treatment following systemic chemotherapy for patients with ES-SCLC (5). More recently, Takahashi et al. reported that PCI did not provide a survival benefit for patients with ES-SCLC (6). While in the latter study, patients underwent baseline magnetic resonance imaging (MRI) of the brain, this was not the case in the former trial; therefore, the concern is that potential treatment of subclinical brain metastases contributed toward an apparent overall survival benefit in the EORTC trial. As a result, there is a need for refinement of criteria in selecting patients with ES-SCLC for PCI following initial systemic therapy.
Recent studies have shown an association between the size of primary tumor and overall extent of disease with an increased rate of brain metastases in patients with LS-SCLC (7,8). For patients with ES-SCLC, it has been reported that patients with less than 5 kg weight loss and good response to chemotherapy were at a higher risk for developing brain metastases (9). Given the lack of consensus on which patients with ES-SCLC to select for PCI, our primary goal was to evaluate clinical factors in an effort to identify patients at an increased risk for brain metastases.
Methods
After obtaining Institutional Review Board approval, we performed a single-institution retrospective review of 173 consecutive patients with ES-SCLC treated between 2010 through 2015. Initial staging assessment was based on MRI, computed tomography (CT), or positron emission tomography (PET)/CT within 3 months of diagnosis. A total of 117 patients were initially diagnosed without brain metastases and received systemic chemotherapy. After excluding patients who received PCI and less than 2 cycles of platinum doublet therapy, 93 patients remained for analysis. Patient records were reviewed for clinical and radiographic features previously identified as relevant baseline risk factors, including age at diagnosis, number and location of metastatic sites defined as sites beyond the primary tumor and regional lymph nodes, T stage, N stage, primary tumor size, performance status, number of systemic therapy cycles, weight loss during chemotherapy, and response to chemotherapy. Response to systemic therapy was assessed by radiographic imaging. Following treatment, a diagnosis of brain metastases was made by either MRI or contrast-enhanced CT.
Statistical analysis
Statistical analysis was performed using SPSS® version 24.0 (IBM, Chicago, IL, USA). The primary outcome was brain metastasis-free survival (BMFS), defined as freedom from brain metastasis or death from time of diagnosis. Actuarial rates of BMFS were estimated using Kaplan-Meier methods, and log-rank tests were employed to assess differences in rates based on individual variables. Pathologic, clinical, and treatment risk factors found to be predictive on univariable analysis (UVA) were included in the Cox multivariable (MVA) regression model. A two-sided model was used for all tests, with an α (type I) error of <0.05 considered to be statistically significant.
Results
Clinical, pathologic and treatment characteristics
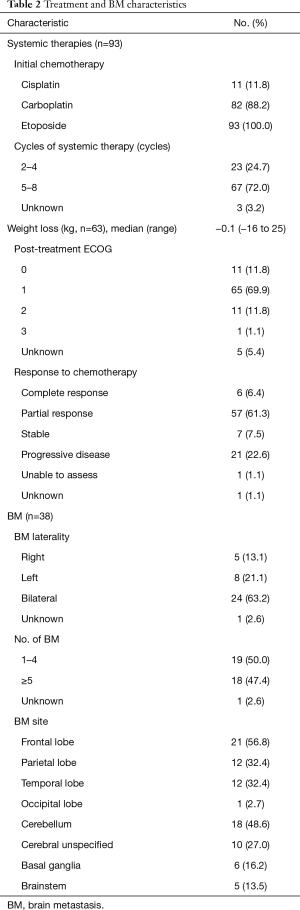
Baseline clinicopathologic characteristics of the cohort (n=93) are shown in Table 1. The median age was 63 years old (range, 39–87 years). There were 39 males (41.9%) and 54 females (58.1%). For the 86 patients in which nodal stage was available, the majority had either N2 or N3 disease (57.0% and 22.6%, respectively). Twenty (21.5%) patients had 3 or more extracranial sites involved with metastatic disease, with the majority of patients having bone (57.0%) and liver metastases (59.1%). All patients received at least 2 cycles of platinum doublet therapy, with the precise number of cycles available for 97% of patients; 67 patients (72.0%) had at least 5 cycles of chemotherapy (Table 2). Response to systemic therapy was available for all but 1 patient; the majority (61.3%) of patients was determined to have a partial response to treatment by radiographic evaluation, while 22.6% showed progression of disease through treatment.

Full table
Full table
BMFS
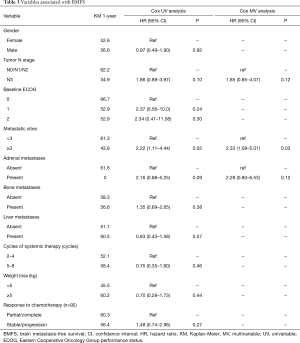
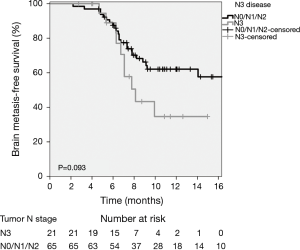
Median follow-up was 10.7 months (range, 3–58 months). Thirty-eight (40.9%) patients developed brain metastases, with a median time to brain metastasis of 8.1 months (range, 2–58 months). Details regarding the number, laterality and site of brain metastases were available for all but 1 patient (Table 2). The majority of patients developed bilateral brain metastases (63.2%), while 18 patients (47.4%) had ≥5 brain metastases. The most common sites of brain metastases were the frontal lobe and cerebellum (56.8% and 48.6% of brain metastases, respectively). Table 3 shows variables associated with BMFS. Involvement of three or more metastatic sites was associated with inferior BMFS on UVA (1-year estimate 43.8% vs. 61.3%; P=0.020) (Figure 1) and MVA [hazard ratio (HR) 2.33, 95% CI: 1.08–5.01; P=0.03]. There was a trend towards inferior BMFS in the presence of N3 when compared with N0/N1/N2 disease on UVA (1-year estimate 34.9% vs. 62.2%; P=0.093) (Figure 2) and MVA (HR 1.85, 95% CI: 0.85–4.07; P=0.12).
Full table

Overall survival
By the time of last follow-up, 80 patients (86.0%) died. There was no significant difference in overall survival between those who developed brain metastases and those who remained free of brain metastases (1-year estimate 47.7% vs. 39.3%; P=0.91).
Restaging CNS imaging
Forty-four (47.3%) patients underwent brain MRI within 60 days of completion of systemic therapy. Twenty-nine (65.9%) were found to have no evidence of brain metastases. Of these patients, 12 (41.4%) went on to develop brain metastases.
Discussion
The 2007 EORTC trial showed that PCI for patients with ES-SCLC decreased the incidence of symptomatic brain metastases and improved disease-free and overall survival (5). The criticism of this study is that patients did not receive routine brain imaging prior to the treatment, and therefore a subgroup of patients may have received treatment for subclinical brain metastases, as opposed to undergoing PCI in the absence of radiographic evidence of brain metastases. In contrast, brain MRI was required after initial platinum doublet therapy in the recent trial published by Takahashi et al. (6). In this trial, only those patients without brain metastases on post-chemotherapy MRI were eligible for randomization; patients were randomized to PCI vs. close surveillance MRI every 3 months. Patients randomized to surveillance imaging without PCI subsequently found to have brain metastases were then treated with salvage whole brain radiotherapy (WBRT). The number of patients developing brain metastases who subsequently received salvage WBRT in surveillance arm of the Japanese trial was higher than the observation arm of the EORTC trial (83% vs. 59%, respectively). Utilization of a post-chemotherapy MRI to exclude patients with radiographic evidence of brain metastases prior to enrollment, in addition to early detection of subclinical brain metastases with close MRI surveillance among patients who enrolled but did not receive PCI, may explain the discordant results between these two randomized studies. The conflicting results from these trials have led to a current controversy as to whether PCI should be offered as standard care for ES-SCLC patients who have responded to initial systemic therapy, or whether they should rather be followed with surveillance imaging and salvage WBRT.
In light of these discrepant phase III trial results and in an effort to assess the role of post-chemotherapy MRI among our patient cohort, we evaluated a relevant subset of patients who underwent brain MRI within 60 days after treatment. Approximately 35% of these patients were found to have evidence of new brain metastases at the time of this scan and most patients found to have brain metastases at this time received subsequent WBRT. Based on this finding in our study and in conjunction with the results from the recently published study from Takahashi et al., we believe that future trials evaluating PCI should include restaging MRI following initial systemic therapy prior to PCI randomization. The proportion of patients with negative brain restaging on post chemotherapy MRI who went on to develop brain metastases (41.4%) was consistent with the overall rate in our study (40.9%). Within our patient cohort, negative post-chemotherapy MRI does not appear to reduce the risk of subsequent brain metastases. Very few patients underwent close MRI surveillance imaging after completion of platinum-based chemotherapy, so it is not possible to know if such a strategy might have improved outcomes for patients compared to a strategy that does not utilize routine MRI surveillance imaging.
Other factors previously reported to predict development of brain metastases were also analyzed in our study. A 2011 retrospective study from Ontario attempted to identify high-risk patients with ES-SCLC who might be better selected to receive PCI; they found that the highest risk cohort to develop brain metastases were patients with weight loss less than 5 kg and a good partial response to upfront chemotherapy (9). These subgroups of patients were not found to be at a significantly higher risk for brain metastases in our analysis. Partial or complete response to chemotherapy (67.7%) in our study was also not predictive of developing brain metastases.
In our study, extent of disease as determined by the number of involved metastatic sites and less significantly, N3 stage, was independently predictive of a diagnosis of brain metastases. While there was a trend towards developing brain metastases in patients with N3 disease, the relatively few number of events and the absence of nodal status in 7 patients in our study may have contributed to the inability to detect a significant difference in this group. Although individual disease sites were not predictive of brain metastases, the cumulative burden and perhaps lymphovascular spread may be associated with seeding of the CNS. A recent retrospective study from Bang et al. showed the presence of extrathoracic metastases to be an independent predictor (HR 2.59) of shorter time to brain metastasis, though the number of metastatic sites was not quantified (10). The theory that extent of disease in patients with SCLC is predictive of brain metastasis is further supported by two recent studies evaluating this relationship in patients with LS-SCLC. A retrospective study by Farooqi et al. identified primary tumors ≥5 cm to be the only independent risk factor (HR 1.66) for development of brain metastases (7). Wu et al. defined LS-SCLC as T3N3M0 disease or lower and found that stage III disease was predictive of brain metastases when compared with stage I/II disease (HR 2.09) (8). These two studies indicate that in LS-SCLC, where PCI is currently standard of care, individualized discussion of whether to deliver PCI for stage I and II patients should be considered.
This study is limited by its retrospective, non-randomized design, timeframe over which it took place and the absence of restaging and surveillance brain imaging in a significant portion of patients. Despite these limitations, it appears that there is increasing evidence that the extent of disease, defined in our study as ≥3 extracranial distant metastatic sites may serve as a surrogate marker predicting for brain metastases and therefore could be used to personalize decision-making for PCI among patients with ES-SCLC. While surveillance imaging with brain MRI might be most appropriate for patients at low risk for developing brain metastases, those patients at highest risk for developing brain metastases may be best served by a strategy utilizing PCI.
Conclusions
Our results suggest that patients with ES-SCLC and three or more extracranial distant metastases demonstrate an increased risk for brain metastases. In the controversial landscape regarding benefit of PCI for this patient population, utilizing this clinical biomarker may assist PCI decision-making on an individual basis. Further validation of this biomarker is necessary to demonstrate its utility and additional approaches aiming to measure disease extent such as quantification of extracranial tumor volume based on CT or PET datasets may offer more robust tools to improve brain metastasis risk estimates.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study has been approved by an institutional review board (approval # Pro00028085); as patient data was de-identified, the study was exempt from requiring written informed consent.
References
- Koinis F, Kotsakis A, Georgoulias V. Small cell lung cancer (SCLC): no treatment advances in recent years. Transl Lung Cancer Res 2016;5:39-50. [PubMed]
- Osterlind K, Hansen HH, Hansen HS, et al. Chemotherapy versus chemotherapy plus irradiation in limited small cell lung cancer. Results of a controlled trial with 5 years follow-up. Br J Cancer 1986;54:7-17. [Crossref] [PubMed]
- Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36-42. [Crossref] [PubMed]
- Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [Crossref] [PubMed]
- Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664-72. [Crossref] [PubMed]
- Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:663-71. [Crossref] [PubMed]
- Farooqi AS, Holliday EB, Allen PK, et al. Prophylactic cranial irradiation after definitive chemoradiotherapy for limited-stage small cell lung cancer: Do all patients benefit? Radiother Oncol 2017;122:307-12. [Crossref] [PubMed]
- Wu AJ, Gillis A, Foster A, et al. Patterns of failure in limited-stage small cell lung cancer: Implications of TNM stage for prophylactic cranial irradiation. Radiother Oncol 2017;125:130-5. [Crossref] [PubMed]
- Greenspoon JN, Evans WK, Cai W, et al. Selecting patients with extensive-stage small cell lung cancer for prophylactic cranial irradiation by predicting brain metastases. J Thorac Oncol 2011;6:808-12. [Crossref] [PubMed]
- Bang A, Kendal WS, Laurie SA, et al. Prophylactic Cranial Irradiation in Extensive Stage Small Cell Lung Cancer: Outcomes at a Comprehensive Cancer Centre. Int J Radiat Oncol Biol Phys 2018;101:1133-40. [Crossref] [PubMed]


