A nomogram for predicting lymph node metastasis in surgically resected T1 esophageal squamous cell carcinoma
Introduction
The management of T1 esophageal carcinoma remains controversial (1-3). Esophagectomy with radical lymphadenectomy have been considered the treatment paradigm for such patients. To achieve a less invasive and better quality of life, endoscopic therapies for T1 esophageal carcinoma have been increasingly used (4,5). And the advanced therapeutic endoscopic techniques can, resection of superficial lesions and ablation of residual mucosa, preserving esophagus without radical resection that performed with lower mortality and morbidity (6,7).
However, the application of these procedures has been limited by without lymph nodes removed, possibility of region lymph node metastasis (LNM) in T1 esophageal carcinoma (8). Due to the abundant lymph-capillary plexus in the lamina propria mucosa and submucosal layer of esophageal, the frequency of LNM is up to 54% in patients with tumors involving submucosal layer (9). Radical lymphadenectomy to harvest all potentially involved nodes is greatly important for curative treatment (10,11). Therefore, it is essential to construct effective model for predicting the risk of LNM before making therapeutic procedures.
The nomogram is reliable as a statistical predictive model which created a simple intuitive graph that accurately clarity the risk of a clinical event (12,13). In present study, we aimed to identify the independent factors that predicted LNM in patients with T1 esophageal squamous cell carcinoma (ESCC). A nomogram model for predicting the potential risk of LNM was then useful to support clinicians in individually therapeutic recommendations.
Methods
Patients
From January 2014 to December 2016, we retrospectively reviewed consecutive patients who underwent esophagectomy with radical lymphadenectomy for ESCC in Shanghai Zhongshan Hospital and Ningbo Medical Center Lihuili Eastern Hospital. The inclusive criteria of our present study were as follows: (I) thoracic T1 ESCC; (II) underwent esophagectomy with radical lymphadenectomy; (III) 12 or more lymph nodes harvested; (IV) no preoperative chemotherapy or radiotherapy. Finally, there were 221 patients met the inclusive criteria, 85 patients from Ningbo Medical Center Lihuili Eastern Hospital and 136 patients from Shanghai Zhongshan Hospital. Analyzed variables included age, gender, tumor location, tumor length, differentiation, lymphovascular invasion and tumor invasion depth. The institutional review board of both hospitals approved the present retrospective study.
The specimens were histopathologically examined and repeatedly reviewed by experienced pathologists. The size of the primary cancer, sample margins, lymphovascular invasion and lymph nodes were assessed. Patients with T1 ESCC were stratified to T1a (tumor invades mucosa) which includes T1a-EP (carcinoma in situ, Tis), T1a-LPM (tumor invades lamina propria mucosa), T1a-MM (tumor invades muscularis mucosa), and T1b which includes SM1(tumor invades the upper third of the submucosal layer), SM2 (tumor invades the middle third of the submucosal layer), SM3 (tumor invades the lower third of the submucosal layer) (14).
Statistical analysis
The linearity assumption in continuous variables was examined with restricted cubic splines. The associations of the risk of LNM in patients with T1 ESCC with clinical characteristics were evaluated using univariate logistic regression analysis. The significant variables with P values less than 0.05 were entered into the multivariate logistic analysis to identify the independent risk factors for LNM. On the basis of results from the multivariable analysis, a nomogram for LNM probability was constructed using a backward step-down process with Akaike information criterion (AIC).
The performance of the nomogram was assessed by discrimination and calibration (15), assessed by receiver operating characteristic (ROC) curve and calibration curves respectively. In addition, the nomogram was subjected to 1,000 bootstrap resamples for internal validation to assess their predictive accuracies.
The statistical analyses were performed using the SAS 9.4 (SAS Institute Inc., Cary, NC, USA). The standard Chi-square test or Fisher’s exact test was used for comparative analysis. Univariate and multivariate logistical regression analyses were performed to predict the risk factors of LNMs. A nomogram, ROC and calibration curves were done with R 3.4.1 (The R Foundation for Statistical Computing, Vienna, Austria). For all the analyses, the results of P<0.05 was considered to be statistically significant.
Results
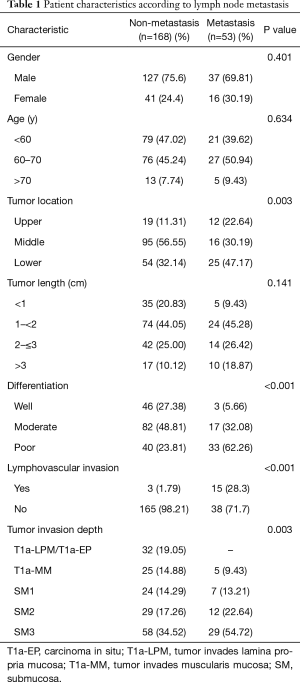
The clinical characteristics of are showed in Table 1. A total of 221 patients were enrolled in his study, 164 males (74.2%) and 57 females (25.8%). All patients performed Mckeown operation with two or three fields lymph node dissection. All patients have curative R0 resection. The median number of lymph nodes harvested was 20 (rang, 12–50), and the frequency of LNM was 24% (53/221). The patients were divided into metastasis group and non-metastasis group. There were significantly different between the two groups in differentiation (P<0.001), lymphovascular invasion (P<0.0001) and tumor invasion depth (P=0.003). In patients with T1 ESCC, no LNMs occurred in patients with T1a-LPM/T1a-EP, but 5 of 30 patients (16.7%) with T1a-MM, 7 of 31 patients (22.6%) with SM1, 12 of 41 patients (29.3%) with SM2, 29 of 87 patients (33.3%) with SM3.
Full table
Independent risk factors for LNM
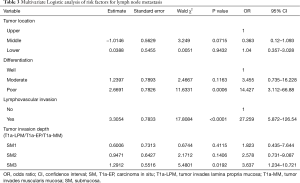
The univariate analysis demonstrated that middle tumor location, tumor length >3 cm, poor differentiation, lymphovascular invasion, SM2 and SM3 were associated with LNM occurrence in T1 ESCC (Table 2). Afterwards, variables of tumor length, tumor location, differentiation, lymphovascular invasion and tumor invasion depth were entered the multivariable logistic regression analysis. The results showed the middle poor differentiation (P=0.0006), lymphovascular invasion (P<0.0001) and SM3 (P=0.0192) were significantly independent risk factors for LNM (Table 3), but tumor length was found no significantly different.

Full table
Full table
Predictive nomogram model for the probability of LNM
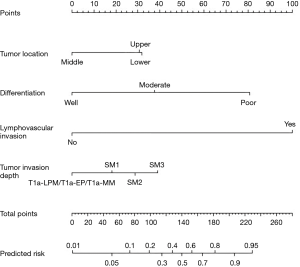
For predicting the risk of LNM, the four significantly independent risk factors were incorporated by constructed a nomogram (Figure 1). A total score was calculated by tumor location, differentiation, lymphovascular invasion and tumor invasion depth. A score was respectively given on the point scale axis. A total score could be easily calculated by adding each single score and, by projecting the total score to the lower total point scale, we were able to predict the probability of LNM.
Performance of the nomogram
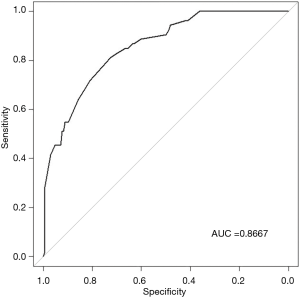
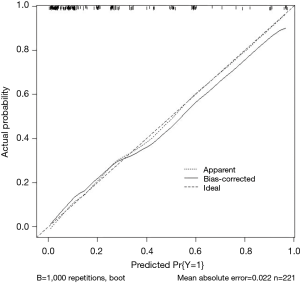
The ROC analysis is showed in Figure 2, which demonstrates nomogram has a robust discrimination, with an area under the receiver operating characteristic curve (AUC) of 0.8667 (Figure 2). According to the calibration curve, the LNM probabilities predicted by the nomogram consisted with the actual probabilities (Figure 3).
Discussion
The management of patients with T1 esophageal carcinoma is controversial (1-3). In present study, we use a simple and intuitive graph of a statistical predictive model which predicting the possibility of LNM and thereby may support theoretical and evidential recommendations to clinicians when making appropriate treatment. We demonstrate that the poor differentiation (P=0.0006), lymphovascular invasion (P<0.0001) and SM3 (P=0.0192) were significantly independent risk factors for LNM.
In our nomogram, specific probabilities of LNM were predicted by optimal discrimination and excellent calibration. Previously, Bin and colleagues constructed a nomogram to predict the risk of LNM in patients with submucosal ESCC, but not assessed by discrimination and calibration (12). The discriminative ability of the nomogram model was determined by the area under the ROC curve, which ranged from 0.5 (no discrimination) to 1 (perfect discrimination) (16). The calibration of the predictive model was performed by a visual calibration plot comparing the predicted and actual probability of LNM (17).
Compared to esophagectomy, endoscopic therapies have the advantages of a less invasive, lower postoperative complications and better quality of life (4,5,7). Ell and colleagues reported that endoscopic therapies in superficial esophageal carcinoma had the results of practically zero mortality and very lower morbidity (7). However, an indiscriminate use of endoscopic therapy may decrease the survival of such patients, because of no lymph nodes removed and possibility of nodal metastasis (18,19). And adjuvant therapies should be offered for a survival benefit in patients with LNM after surgical pathologic examination (20). Therefore, it is necessary to understand the prevalence and risk of LNM in patients with T1 esophageal carcinoma (21,22).
Our analysis of population-based date shows that the prevalence of LNM is relatively high: about 24% of all patients with surgically resected T1 ESCC suffered LNM. We have found the frequency of LNM was 8.1% in patients with intramucosal cancer (no LNM in T1a-LPM/T1a-EP), and 30.3% in patients with submucosal cancer. Results of prevalence of LNM were generally consistent with previous studies (12,23,24). Some studies demonstrated there was no risk of LNM in intramucosal cancer (25,26). Nowadays, the diagnostic procedures and immunohistochemical predictors are unreliable for predicting nodal metastasis (27-29). In our nomogram, the lymphovascular invasion is the greatest contributor to the risk of LNM, followed by differentiation and tumor invasion depth. Tumor location was the smallest effect on the risk of LNM.
Several limitations in our study should be addressed. First of all, we analyzed data only from the patients who underwent surgically resected T ESCC, patients who not undergo a resection were excluded, result in selective bias. In addition, considering the differences in epidemiology and clinical behavior that exist between ethnic groups, the generalizability of this nomogram still requires external validation using additional databases (30). Finally, our predictive model is constructed by retrospective data and the results should been validated in another population.
In conclusion, our results show the middle tumor location, poor differentiation, lymphovascular invasion and SM3 were significantly independent risk factors for LNM. The nomogram model is greatly convenient, highly accurate, excellently calibrated. This nomogram might usefully help clinicians to make individualized predictions of each patient’s probability of LNM and to improve treatment recommendations for patients with a T1 esophageal carcinoma.
Acknowledgements
Funding: Supported by the National Natural Science Foundation of China (Grant No. 81400681) and the Ningbo Medical Center Lihuili Eastern Hospital (Grant No. 2017DYKY05).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of The Zhongshan Hospital and Ningbo Medical Center Lihuili Eastern Hospital (No. 2017236).
References
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012;143:336-46. [Crossref] [PubMed]
- Molena D, DeMeester SR. The dilemma of T1 esophageal adenocarcinoma. J Thorac Cardiovasc Surg 2017;153:1206-7. [Crossref] [PubMed]
- Louie BE. To resect or not to resect: The decision in T1 esophageal adenocarcinoma. J Thorac Cardiovasc Surg 2017;153:1208. [Crossref] [PubMed]
- Bennett C, Green S, Decaestecker J, et al. Surgery versus radical endotherapies for early cancer and high-grade dysplasia in Barrett’s oesophagus. Cochrane Database Syst Rev 2012;11. [PubMed]
- Ishihara R, Iishi H, Uedo NT, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008;68:1066-72. [Crossref] [PubMed]
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652-60.e1. [Crossref] [PubMed]
- Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc 2007;65:3-10. [Crossref] [PubMed]
- Dubecz A, Kern M, Solymosi N, et al. Predictors of Lymph Node Metastasis in Surgically Resected T1 Esophageal Cancer. Ann Thorac Surg 2015;99:1879-85; discussion 1886.
- Eguchi T, Nakanishi Y, Shimoda T, et al. Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: analysis of 464 surgically resected cases. Mod Pathol 2006;19:475-80. [Crossref] [PubMed]
- Grotenhuis BA, van Heijl M, Zehetner J, et al. Surgical management of submucosal esophageal cancer:extended or regional lymphadenectomy? Ann Surg 2010;252:823-30. [Crossref] [PubMed]
- Dutkowski P, Hommel G, Böttger T, et al. How many lymph nodes are needed for an accurate pN classification in esophageal cancer? Evidence for a new threshold value. Hepatogastroenterology 2002;49:176-80. [PubMed]
- Li B, Chen H, Xiang J, et al. Prevalence of lymph node metastases in superficial esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2013;146:1198-203. [Crossref] [PubMed]
- Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861-9. [Crossref] [PubMed]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer (11th Edition: part I) Esophagus 2017;14:1-36.
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-70. [Crossref] [PubMed]
- Bandos AI, Rockette HE, Song T, et al. Area under the free-response ROC curve (FROC) and a related summary index. Biometrics 2009;65:247-56. [Crossref] [PubMed]
- Harrell FE Jr, Lee KL, Mark DB, et al. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87. [Crossref] [PubMed]
- Ozawa Y, Kamei T, Nakano T, et al. Characteristics of Postoperative Recurrence in Lymph Node-Negative Superficial Esophageal Carcinoma. World J Surg 2016;40:1663-71. [Crossref] [PubMed]
- Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815-23. [Crossref] [PubMed]
- Samson P, Puri V, Lockhart AC, et al. Characteristics and outcomes of pathological node positive esophageal cancer patients receiving adjuvant chemotherapy following induction chemotherapy and esophagectomy. Presented at: 95th Annual Meeting of The American Association for Thoracic Surgery; May 14-18, 2016; Baltimore, MD.
- Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol 2013;11:630-5. [Crossref] [PubMed]
- Boys JA, Worrell SG, Chandrasoma P, et al. Can the risk of lymph node metastases be gauged in endoscopically resected submucosal esophageal adenocarcinomas? A multi-center study. J Gastrointest Surg. 2016;20:6-12; discussion 12. [Crossref] [PubMed]
- Altorki NK, Lee PC, Liss Y, et al. Multifocal neoplasia and nodal metastases in T1 esophageal carcinoma: implications for endoscopic treatment. Ann Surg 2008;247:434-9. [Crossref] [PubMed]
- Pennathur A, Farkas A, Krasinskas AM, et al. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg 2009;87:1048-54; discussion 1054-5. [Crossref] [PubMed]
- Bollschweiler E, Baldus SE, Schröder W, et al. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy 2006;38:149-56. [Crossref] [PubMed]
- Ancona E, Rampado S, Cassaro M, et al. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol 2008;15:3278-88. [Crossref] [PubMed]
- Pech O, May A, Günter E, et al. The impact of endoscopic ultrasound and computed tomography on the TNM staging of early cancer in Barrett’s esophagus. Am J Gastroenterol 2006;101:2223-9. [Crossref] [PubMed]
- Pouw RE, Heldoorn N, Alvarez Herrero L, et al. Do we still need EUS in the workup of patients with early esophageal neoplasia? A retrospecttive analysis of 131 cases. Gastrointest Endosc 2011;73:662-8. [Crossref] [PubMed]
- Dhupar R, Rice RD, Correa AM, et al. Endoscopic ultrasound estimates for tumor depth at the gastroesophageal junction are inaccurate: implications for the liberal use of endoscopic resection. Ann Thorac Surg 2015;100:1812-6. [Crossref] [PubMed]
- Zhou W, Christiani DC. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer 329 between East Asians and Caucasians. Chin J Cancer 2011;30:287-92. [Crossref] [PubMed]


