Application of ex vivo lung perfusion (EVLP) in lung transplantation
Lung transplantation is the ultimate effective treatment for benign end-stage lung disease. Dr. Hardy performed the first lung transplant in a human in 1963 (1), and the patient survived 18 days after operation. Approximately 40 lung transplants were performed during the following two decades, but the postoperative survival rate was extremely low. Most patients died of immune rejection, infection, or anastomotic complications. With the clinical application of immunosuppressants such as cyclosporine, the Toronto Lung Transplant Group first successfully performed single-lung transplantation in 1983 (2) and double-lung transplantation in 1986 (3). Since then, lung transplantation has been rapidly progressing. According to the International Society for Heart and Lung Transplantation (ISHLT) registry, 3,893 lung transplants were performed and registered worldwide in 2013, indicating considerable progress in lung transplantation. Currently, donor shortage remains a major limitation of lung transplantation. The utilization rate of donor lungs is significantly lower than that of other solid organs. Studies (4) have shown that only 15–20% of donor lungs are effectively utilized, while the rate is 30% for donor hearts. The main reasons for this low rate are related to complications such as pulmonary contusion, aspiration, mechanical ventilation pressure injury, ventilator-associated pneumonia, and neurogenic pulmonary edema. However, according to the reports, approximately 40% of discarded donor lungs are still usable (5,6). In recent years, the number of patients waiting for a lung transplant has been increasing annually, while the death rate among those on the waiting list has also increased because of the donor shortage. Several measures that expand the availability of donor lungs have been used in clinical practice, such as the use of marginal donors, donors of cardiac death, lobar donors for recipients with a small thoracic cavity, and extracorporeal membrane oxygenation (ECMO) support for donor lungs with compromised quality. Ex vivo lung perfusion (EVLP) is a technique used to evaluate and screen compromised donor lungs with potential for recovery. This technique has been already used in lung transplantation centers in North America and Europe.
Brief history of the development of EVLP
Carrel and Lindbergh first proposed the idea of ex vivo organ perfusion in 1935 (7). They removed the thyroids of cats and rabbits for ex vivo perfusion for approximately one week. EVLP was initially proposed by Hardesty (8), but the idea was abandoned because of unsatisfactory results. In the 1990s, Steen et al. (9,10) evaluated lung functions with EVLP and then later reported the preliminary results of a series of pivotal cases of EVLP; in 2000, EVLP was used to evaluate a lung from a non-heart-beating donor before lung transplantation; in 2005, the team performed EVLP to re-evaluate a lung considered ineligible during initial evaluation, and the lung was finally transplanted successfully. These results provided initial experiences for subsequent clinical application of EVLP. In 2006, Wierup et al. (11) built upon Steen’s experience and conducted a clinical study. The results showed that in six cases, donor lungs were considered ineligible during initial evaluation, re-evaluated and deemed eligible with EVLP, and successfully transplanted. In 2009, the Toronto Lung Transplant Group (12) proposed the Toronto EVLP protocol; in 2011 (13), they published an article in the New England Journal of Medicine reporting that in 20 cases, “ineligible” donor lungs were re-evaluated with EVLP and then successfully transplanted. This study showed that using EVLP is feasible in clinical practice.
Mechanism of action and indications for EVLP
EVLP can restore the circulation and ventilation of the ex vivo lung. At an ambient temperature of 37 ℃, a membrane oxygenator is used to simulate oxygen consumption in the body via deoxygenation and maintain the physiological state of lungs with specific perfusate and ventilation. The Steen solution is currently the only Food and Drug Administration (FDA)-approved EVLP perfusate for clinical use. The ventilation gas in the lung membrane consists of N2 (86%), CO2 (8%), and O2 (6%). The hypoxic gas mixture removes the oxygen in the circuit to simulate oxygen consumption in the body. The EVLP system includes a ventilator, an endotracheal tube, perfusate and a fluid circuit, a reservoir, an oxygenator, a pump, and a thermostat.
EVLP is currently used mainly to evaluate certain high-risk donor lungs. It is mainly indicated for (13,14): (I) an oxygenation index <300 mmHg; (II) pulmonary edema as indicated by the last chest X-ray; (III) collapse or poor expansion of a donor lung during harvest; (IV) blood transfusion >10 U; and (V) lungs from donors with cardiac death. EVLP is not suitable in cases of apparent pneumonia, severe mechanical lung injury (including multiple lobar injury), or significant aspiration of gastric contents.
After EVLP, a donor lung is considered eligible for transplant if (13,14) the oxygenation index reaches 400 mmHg after 4–6 hours of EVLP; chest X-ray findings are stable or improved; and pulmonary artery pressure, airway pressure, and lung compliance are stable or improved. A reconditioned donor lung is considered ineligible for transplant if the oxygenation index is <400 mmHg; pulmonary arterial pressure, airway pressure, or lung compliance worsens by ≥15% from baseline; and chest X-ray shows worsening signs.
The primary types of EVLP
Currently, three EVLP systems (Table 1) are commercially available for clinical use: the Toronto system, the Lund system, and the Organ Care System (OCS). The Toronto system is the most widely used system. The Lund system is an extension of the original EVLP protocol. The OCS is currently the only portable EVLP system. For both the Toronto and Lund systems, the donor lung is cryopreserved after harvest and during transportation and is connected to the EVLP device for perfusion at ambient temperature after it is delivered to the recipient’s hospital. The transportation time is counted towards the cold ischemia time. On the other hand, the OCS allows the donor lung to be immediately connected to the EVLP system for perfusion at ambient temperature after cold perfusion and harvest, thereby reducing the cold ischemia time.
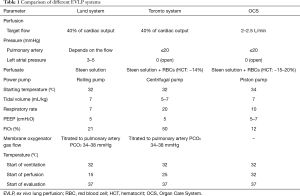
Full table
During EVLP and evaluation, the graft can be examined in detail by direct touch, bronchoscopy, and imaging studies to rule out tumors, pulmonary contusion, pulmonary edema, infection, embolism, and interstitial lung disease. Pulmonary function evaluation includes blood gas analysis, hemodynamics, and mechanical ventilation parameters over several hours. During this time, lung tissue specimens and bronchoalveolar lavage fluid can be tested for microbial, molecular, and histomorphological markers, which help determine the quality of a donor lung.
Clinical application of EVLP
Currently, EVLP is mainly used to evaluate the quality of a donor lung at large lung transplant centers in North America and Europe. In 2011, the results of the first prospective clinical trial “HELP” (13) showed that 20 of 23 high-risk donor lungs proceeded to transplantation after evaluation; in the control group, 116 lung transplants were performed after conventional standard screening of donor lungs. No significant difference was observed in primary graft failure, the length (days) of postoperative mechanical ventilation, ICU stay (days), hospital stay (days), or 30-day mortality. Aigner et al. (15) reported nine cases of double-lung transplantation after EVLP evaluation. No significant difference was observed between these nine cases and the 119 cases in the control group (conventional method) in mechanical ventilation, ICU stay (days), hospital stay (days), or 30-day mortality. In the FDA-approved multi-center Novel lung trial, 42 of 76 donor lungs ultimately proceeded to transplantation after EVLP evaluation. No significant difference was observed between these cases and the 42 cases in the control group (conventional method) in early outcomes or 1-year survival. Fisher et al. (16) reported the preliminary results of the DEVELOP-UK trial, a non-randomized trial that investigated transplant outcomes of expanded versus standard donors. The results showed that 18 of 53 (34%) lungs from expanded donors were transplanted after EVLP evaluation, with a slightly lower 1-year survival rate than that for lungs from standard donors (n=184), although the difference did not reach statistical significance. Moreover, early graft injury and unscheduled ECMO support rates and medical expenses were higher in the EVLP group. The OCS is a portable EVLP device that allows immediate lung perfusion at ambient temperature after harvest, thereby minimizing the cold ischemia time. Luc et al. (17) conducted a phase III study in 151 cases using the OCS versus 169 cases in the control group. The results showed that the 30-day survival rate was 95.7% versus 100%, the 12-month survival rate was 89.4% versus 88.1%, and 72-hour primary graft failure rate was 17.7% versus 29.7%, respectively (P=0.015). The investigators concluded that EVLP reduced 72-hour primary graft failure and may accelerate postoperative recovery and extend long-term survival.
Animal studies on EVLP
Large animals
Advantages: (I) larger size and weight. Large animals such as pigs are advantageous for EVLP studies as experimental parameters, after modification, can be directly applied to human subjects. The size and weight of pigs are similar to those of humans; therefore, the pig is an effective animal model for human conditions. For example, proper tidal volume, positive end-expiratory pressure (PEEP), and perfusion time settings can be used as a basis for clinical trials. In addition, device design and perfusate volume in porcine studies can be used directly in clinical trials, which is not feasible with small animals; (II) similar immune system and physiological environment. The genetic sequence and physiological environment of pigs are closer to those of humans and can better simulate human conditions compared to rats and mice; therefore, the porcine model is an ideal animal model for pre-clinical studies.
Disadvantages: large animals have certain disadvantages, such as being expensive, time-consuming, and labor-intensive, especially with regard to experimental equipment, the perfusate volume required, and labor costs associated with surgical procedures, anesthesia, and experimental management, thus complicating repeat experiments with large animals for validation.
Small animals
Advantages: EVLP studies have been conducted in small animals such as rats, mice, guinea pigs, and rabbits. The experiments are less expensive than those with large animals, which is the greatest advantage of using small animals. In addition, the experiment can be performed by one person, saving time and labor and facilitating repeat experiments as needed for validation. Of the four animals mentioned above, mice are the smallest and most difficult to operate on, while the other three small animals can be operated on without a microscope. Currently, the success rate of lung transplantation in rats is approximately 95%. Fewer studies have been conducted in rabbits and guinea pigs, and most studies involve the ischemia-reperfusion model. Mice are the most difficult to operate on, with a high incidence of accidental injury, but they are a more valuable research tool than rats because of the availability of a large number of protein antibodies and gene probes.
Disadvantages: small animals have certain disadvantages. Rats and mice have a shorter perfusion time. Studies have shown that the perfusion time is 15 minutes to several hours in rats depending on many factors. Studies of lung injury models show that the degree of lung injury after ventilation/perfusion for 15 minutes in rats is similar to that after 4–24 hours in pigs/humans. Moreover, rodent studies have shown a high incidence of pulmonary atelectasis, which, along with airway fluid after lung recruitment, can damage the alveolar epithelium (18,19). In studies with large animals, the cause of atelectasis can be identified, and airway fluid can be removed via bronchoscopy.
Perfusion technique in animal studies
- Perfusate: the Steen solution is commercially available and the most commonly used perfusate. Studies have shown that highly permeable and albumin-containing perfusate is best. Cell-free perfusate does not introduce foreign antigens, eliminating the possibility of red blood cell (RBC) dissolution during perfusion. Moreover, perfusate provides various basic substances for lung metabolism. The lung itself can provide oxygenation, and thus perfusate can be oxygen-free, although it must contain glucose and various electrolytes;
- Ventilation parameters: during EVLP, mechanical ventilation should be protective in nature. For large animals, the tidal volume is usually 4–6 mL/kg and may be as high as 10 mL/kg; ~4 mL/kg is usually used in rats, and significant lung injury occurs at 10 mL/kg;
- Temperature: in general, the temperature should slowly recover during perfusion, usually to 37 ℃ over approximately 30 minutes;
- Perfusion time: studies have shown that the EVLP time is 30 minutes to 3 hours in small animals and up to 14 hours in pigs. EVLP time is related to perfusion settings and animal models;
- Pulmonary arterial perfusion flow and pressure: the goal of pulmonary arterial perfusion flow is to reach a certain level of pulmonary artery pressure or pulmonary vascular resistance. Typically, perfusion flow is incrementally increased to the target flow during the first 15–30 minutes of perfusion. The target flow is 40% of the cardiac output for large animals and humans and ~20% for small animals (20). Pulmonary vascular resistance is calculated with pulmonary artery pressure and flow. If lung function is normal during perfusion, then pulmonary vascular resistance will gradually decrease during perfusion, and increased resistance indicates progressively impaired oxygenation.
The main role of EVLP animal models
Although EVLP technology has been introduced in clinical practice, further experimental studies still rely on dependable animal models.
- Organ function evaluation: during EVLP, the physiological function of the lung can be monitored, including pulmonary artery flow, pulmonary artery pressure, pulmonary vascular resistance, the perfusate oxygen content before and after perfusion. These are effective indicators of lung function;
- The experimental platform for lung injury: EVLP controls ventilation, allowing the use of animal models for ventilator-associated lung injury, the mechanism of which can be studied by accurately adjusting parameters such as tidal volume and PEEP. Many animal models of acute lung injury in vivo, such as loss of surfactant activity, gastric acid-induced lung injury, and lipopolysaccharide (LPS)-induced lung injury, can be used in EVLP models;
- Experimental recovery platform and the route of drug administration: first, the perfusate is modified and optimized to identify optimal conditions and parameters for preserving a donor lung. In addition, more routes of drug administration are used during EVLP such that drugs may be added to the perfusate or mechanical ventilation gas to allow a donor lung to recover. This is an important direction for future studies on donor lung recovery. Cosgun et al. (21) conducted a study in pigs and added trimetazidine to the perfusate in the experimental group. The results showed that after 4 hours of EVLP, oxygenation indicators were superior in the experimental group compared to those in the control group. Francioli et al. (22) added the antioxidant pyrrolidine dithiocarbamate to the perfusate and found that NF-KB was significantly inhibited, pulmonary edema and protein levels in bronchoalveolar lavage fluid were significantly reduced, and TNF-a and IL-6 levels were significantly lower in the experimental group. Currently, most studies on EVLP lung recovery involve adding drugs to the perfusate, which allows the drugs to directly contact the lung during EVLP, thereby enabling better lung recovery. Hijiya et al. (23) conducted a study in dogs with cardiac death. During EVLP of donor lungs, a high-concentration, short-acting β-2 agonist was inhaled 4 times during ventilation. The results showed that oxygenation, lung compliance, pulmonary vascular resistance, and pulmonary edema were significantly better in the experimental group than those in the control group. These animal studies provide a reference for good study designs for future studies on donor lung recovery. EVLP can be used to recover and evaluate donor lungs before transplantation, minimizing adverse effects on the recipient’s quality of life associated with transplantation of an ineligible donor lung.
EVLP is an emerging, revolutionary technology that plays a very important role in donor lung recovery and evaluation. It also helps expand the availability of donor lungs and alleviates the shortage of lung transplant donors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hardy JD, Webb WR, Dalton ML Jr, et al. Lung Homotransplantation in Man. JAMA 1963;186:1065-74. [Crossref] [PubMed]
- Toronto Lung Transplant Group. Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med 1986;314:1140-5. [Crossref] [PubMed]
- Patterson GA, Cooper JD, Dark JH, et al. Experimental and clinical double lung transplantation. J Thorac Cardiovasc Surg 1988;95:70-4. [PubMed]
- Punch JD, Hayes DH, LaPorte FB, et al. Organ donation and utilization in the United States, 1996-2005. Am J Transplant 2007;7:1327-38. [Crossref] [PubMed]
- Van Raemdonck D, Neyrinck A, Verleden GM, et al. Lung donor selection and management. Proc Am Thorac Soc 2009;6:28-38. [Crossref] [PubMed]
- Ware LB, Wang Y, Fang X, et al. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet 2002;360:619-20. [Crossref] [PubMed]
- Carrel A, Lindbergh CA. The Culture of Whole Organs. Science 1935;81:621-3. [Crossref] [PubMed]
- Hardesty RL, Griffith BP. Autoperfusion of the heart and lungs for preservation during distant procurement. J Thorac Cardiovasc Surg 1987;93:11-8. [PubMed]
- Steen S, Sjoberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet 2001;357:825-9. [Crossref] [PubMed]
- Steen S, Ingemansson R, Eriksson L, et al. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg 2007;83:2191-4. [Crossref] [PubMed]
- Wierup P, Haraldsson A, Nilsson F, et al. Ex vivo evaluation of nonacceptable donor lungs. Ann Thorac Surg 2006;81:460-6. [Crossref] [PubMed]
- Cypel M, Rubacha M, Yeung J, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant 2009;9:2262-9. [Crossref] [PubMed]
- Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. [Crossref] [PubMed]
- Machuca TN, Cypel M. Ex vivo lung perfusion. J Thorac Dis 2014;6:1054-62. [PubMed]
- Aigner C, Slama A, Hotzenecker K, et al. Clinical ex vivo lung perfusion--pushing the limits. Am J Transplant 2012;12:1839-47. [Crossref] [PubMed]
- Fisher A, Andreasson A, Chrysos A, et al. An observational study of Donor Ex Vivo Lung Perfusion in UK lung transplantation: DEVELOP-UK. Health Technol Assess 2016;20:1-276. [Crossref] [PubMed]
- Luc JG, Bozso SJ, Freed DH, et al. Successful repair of donation after circulatory death lungs with large pulmonary embolus using the lung organ care system for ex vivo thrombolysis and subsequent clinical transplantation. Transplantation 2015;99:e1-2. [Crossref] [PubMed]
- Ghadiali SN, Gaver DP. Biomechanics of liquid-epithelium interactions in pulmonary airways. Respir Physiol Neurobiol 2008;163:232-43. [Crossref] [PubMed]
- Yalcin HC, Hallow KM, Wang J, et al. Influence of cytoskeletal structure and mechanics on epithelial cell injury during cyclic airway reopening. Am J Physiol Lung Cell Mol Physiol 2009;297:L881-91. [Crossref] [PubMed]
- Nelson K, Bobba C, Eren E, et al. Method of isolated ex vivo lung perfusion in a rat model: lessons learned from developing a rat EVLP program. J Vis Exp 2015;(96).
- Cosgun T, Iskender I, Yamada Y, et al. Ex vivo administration of trimetazidine improves post-transplant lung function in pig model. Eur J Cardiothorac Surg 2017;52:171-7. [Crossref] [PubMed]
- Francioli C, Wang X, Parapanov R, et al. Pyrrolidine dithiocarbamate administered during ex-vivo lung perfusion promotes rehabilitation of injured donor rat lungs obtained after prolonged warm ischemia. PLoS One 2017;12. [Crossref] [PubMed]
- Hijiya K, Chen-Yoshikawa TF, Kondo T, et al. Bronchodilator Inhalation During Ex Vivo Lung Perfusion Improves Posttransplant Graft Function After Warm Ischemia. Ann Thorac Surg 2017;103:447-53. [Crossref] [PubMed]


