Application of albumin/globulin ratio in elderly patients with acute exacerbation of chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease characterized by persistent airflow limitation and mainly occurs in the elderly population. Acute exacerbation of COPD (AECOPD) is an acute event characterized by a worsening of the patient’s respiratory symptoms that are beyond normal day-to-day variations and leads to a change in medication (1). AECOPD has become an important disease of hospitalized elderly patients. In 2020, COPD will represent the fifth largest disease burden worldwide and the third largest cause of death worldwide (2). The occurrence and development of COPD are associated with airway inflammation and systemic immune disorders (2). The main protein components of human serum albumin, globulin, C-reactive protein (CRP), and prealbumin (PA) play an important role in immunity and inflammation throughout the body (3). The albumin/globulin ratio (AGR) can be calculated by measuring the total serum protein and albumin concentrations. The AGR can be used for prognostic evaluation of tumors (4,5). Although serum albumin and globulin were thought to correlate with AECOPD progression, few reports have described the application of the AGR to AECOPD (3,6). This study was performed to assess the relationship between the AGR and AECOPD in elderly patients and explore the application value of the AGR in AECOPD assessment, progression, and prognosis in the elderly population.
Methods
Study population
We retrospectively analyzed the data of patients with COPD in the Department of Respiratory Medicine, First Affiliated Hospital, Guangxi Medical University, China from January 2014 to September 2016. The inclusion criteria of AECOPD patients were: diagnosed with COPD according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria; presentation of an acute exacerbation (increased dyspnoea, increased sputum purulence, increased sputum volume); age ≥60 years, and were able to tolerate pulmonary function tests; the results of relevant laboratory tests can be inquired in their medical records. The exclusion criteria were heart, liver, kidney, brain and other important organ diseases; autoimmune disease; and malignant tumors. After screening, 252 AECOPD patients were enrolled (228 men and 24 women; age range, 60–96 years; mean age, 71.76±9.36 years). In accordance with the pulmonary function classification, AECOPD patients were assigned to GOLD I, II, II, and IV groups. Infection was present in 136 AECOPD patients and absent in 116. Of the 136 AECOPD patients with infection, 50 (36.76%) patients were bacterial infection, 14 (10.29%) patients were virus infection, 31 (22.79%) patients were atypical pathogen infection, 4 (2.94%) patients were fungal infection and 37 (27.21%) cases were mixed infection. The inclusion criteria of stable COPD patients were: diagnosed with COPD according to the GOLD criteria: age ≥60 years; without acute exacerbation in the last 6 months; results of relevant laboratory tests can be inquired in their medical records. The exclusion criteria were the same as AECOPD. After screening, 89 stable COPD patients were enrolled (80 men and 19 women, age range, 60–93 years; mean age, 70.23±6.12 years). The healthy control group comprised persons undergoing a physical examination at the Medical Examination Center of our hospital during the same period. The inclusion criterion for the control group was an age of ≥60 years. The exclusion criteria were respiratory system diseases, cardiovascular and cerebrovascular diseases; and dysfunction of important organs including the heart, liver, kidney, and brain; malignant tumors; autoimmune diseases; and a history of medical treatment in the past 3 months. In total, 115 controls were enrolled (103 men and 12 women; age range, 60–99 years; mean age, 71.23±5.46 years). This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University, China (No. 2018-KY-E-020).
Study methods
AECOPD patients presenting with all of the following criteria were divided into the with infection group (7): they met the clinical feature for respiratory infection; and were diagnosed with lung inflammation by pulmonary iconography; the results of sputum culture or serological examination were positive. The body mass index (BMI) was equal to weight (kg)/height (m2). Pulmonary function was determined using the MasterScree PFT Pulmonary Function Testing System (JAEGER, Bavaria, Germany). Quality control reached the American thoracic society standard. No bronchodilators were used for 24 hours before testing. Bronchodilator testing was conducted after inhaling 400 µg of salbutamol for 20 minutes. Laboratory indicators included the serum CRP, albumin, total protein, immunoglobulin G (IgG), and PA concentrations. Fasting venous blood (5 mL) was obtained in the morning and centrifuged, serum CRP, albumin, total protein, IgG, and PA levels of patients were examined at the first day of hospitalization. Serum was analyzed with a 7,600 automatic biochemical analyzer (Hitachi, Tokyo, Japan), and the AGR was calculated as follows: AGR = albumin/(total protein—albumin).
Statistical analysis
Data were analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Since the raw CRP data were asymmetrical, the logarithmic transformation (LogCRP) was used to obtain a normal distribution. Measurement data are expressed as the mean ± standard deviation. Differences between groups were compared using an independent sample t-test, and differences among groups were compared using one-way analysis of variance. Unidirectionally ordered measurement data were compared among the groups using a nonparametric rank sum test (Kruskal-Wallis test). The optimal cutoff value of each index was analyzed by a receiver operating characteristic curve. The corresponding value was obtained at the maximum Youden index. After grouping according to the optimal cutoff values, the presence of infection among the groups was compared using the chi-square test. The relationship between the AGR and hospital stay (day) was analyzed using Pearson correlation analysis. A P value of <0.05 was considered statistically significant.
Results
Comparison of each index among AECOPD patients, stable COPD patients, and healthy controls
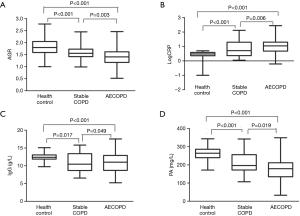
The AGR, IgG and PA were significantly lower in patients with stable COPD and AECOPD than in healthy controls (P<0.001), but logarithm of the serum C-reactive protein (LogCRP) was higher in patients with stable COPD and AECOPD than in healthy controls (P<0.001). The LogCRP (P=0.006) and IgG (P=0.049) were higher in AECOPD than in stable COPD, but the AGR (P=0.003), and PA (P=0.019) were lower in AECOPD than in stable COPD (Figure 1).
Comparison of each index in patients with different GOLD stages
Among the patients with AECOPD, the AGR progressively decreased as the pulmonary function classification increased (P<0.001). The PA level (P<0.001) and LogCRP (P=0.024) were significantly different but the IgG level (P=0.450) was not significantly different among patients with different GOLD stages (Table 1).

Full table
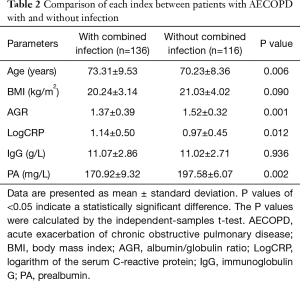
Comparison of each index between AECOPD patients with and without infection
Patients with infection were older than those without infection (P=0.006). The AGR (P=0.001) and PA level (P=0.002) were lower in those with than without infection. LogCRP was higher in patients with than without infection (P=0.012). The IgG level (P=0.936) and BMI (P=0.090) were not significantly different between the two groups (Table 2).
Full table
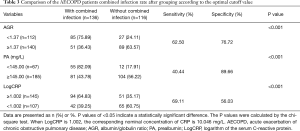
Use of AGR, PA, and LogCRP to determine whether AECOPD is combined with infection in the elderly population
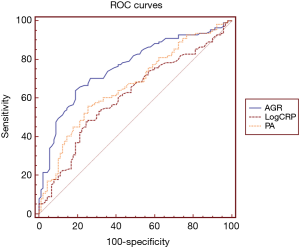
The optimal cutoff values of the AGR, LogCRP, and PA level were calculated by the receiver operating characteristic curve for determining whether AECOPD was associated with infection. The optimal cutoff value of the AGR was 1.37, and area under the curve (AUC) was 0.718 [95% confidence interval (CI), 0.647–0.789; P<0.001]. The optimal cutoff value of the PA level was 145.00 mg/L, and the AUC was 0.632 (95% CI, 0.555–0.709; P=0.001). The optimal cutoff value of LogCRP was 1.002 (10.046 mg/L), and the AUC was 0.618 (95% CI, 0.550–0.685; P=0.001) (Figure 2). After grouping according to the optimal cutoff value, the chi-square test results demonstrated that the combined infection rate was different in elderly patients with AECOPD with an AGR of <1.37 versus ≥1.37 (P<0.001) and between those with a PA level of <145.00 and ≥145.00 mg/L (P<0.001). The combined infection rate was higher in patients with a LogCRP of ≥1.002 than <1.002 (P<0.001). High sensitivity and specificity were obtained when 1.37 was used as the optimal cutoff value of AGR for determining the presence of infection (sensitivity, 62.50%; specificity, 76.72%) (Table 3).
Full table
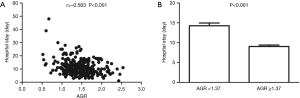
Relationship between AGR and hospital stay
The Pearson correlation analysis results demonstrated that the AGR was negatively correlated with the hospital stay (r=−0.583, P<0.001) (Figure 3). After grouping according to the optimal cutoff value of the AGR (1.37), the hospital stay was significantly longer in patients with an AGR of <1.37 than ≥1.37 (P<0.001) (Figure 3).
Discussion
Elderly individuals are the main population affected by COPD (2). With the gradual development of population aging, the incidence and mortality of COPD have been increasing annually (2). Frequent attacks of acute exacerbation can accelerate the progressive decline of pulmonary function, lower the patient’s quality of life, increase the mortality rate, and result in a heavier social and economic burden (2). Although pulmonary function testing is important in the diagnosis and continued assessment of AECOPD, such testing is not yet available in primary hospitals, and elderly patients’ tolerance to the test is poor.
The AGR is a simple indicator of biochemical liver function and is mainly affected by the serum albumin and globulin concentrations. The AGR can be used as an index for predicting mortality after myocardial infarction (8). Pal et al. (9) indicated that the AGR could be used for the early diagnosis of gestational hypertension. In the present study, the AGR was associated with the occurrence and development of AECOPD. A higher pulmonary function classification was associated with a lower AGR. The AGR can be utilized as an index with which to evaluate the severity of AECOPD. We found that stable COPD patients and AECOPD patients had lower AGR levels than healthy controls. We suspect that there are some causes of changes in the AGR in patients with COPD. One cause is chronic inflammation. Small airway inflammation is a major lesion associated with COPD and an important cause of progressive lung damage; accordingly, we found that CRP and PA levels became remarkably altered with aggravation of lung function damage. Cornwell et al. (10) demonstrated that neutrophils and lymphocytes are the main cells infiltrating the airway walls of patients with COPD. The AGR is associated with neutrophils and lymphocytes (11). IgG is the main component of globulin. We found that IgG in stable COPD patients and AECOPD patients were lower than that in healthy controls, which may be associated with a disorder of immune function in patients with COPD (12). In the present study, IgG in patients with AECOPD is higher than that in stable COPD patients, which may be one cause of AGR in AECOPD patients lower than that in stable COPD patients. Feghali-Bostwick et al. (13) demonstrated that COPD patients had massive deposition of IgG during acute exacerbation, which may be the reason for the higher level of globulin in AECOPD patients than stable COPD patients. The other cause of changes in the AGR is hypoalbuminemia, which is common in patients with COPD. A previous study showed that 21.35% of elderly patients with COPD also had hypoalbuminemia (14). A national cohort study revealed that a higher age is associated with greater declines in the albumin level and more complications and that a low albumin concentration is a risk factor for acute respiratory failure in patients with COPD (15). Finally, we suspect that the administration of drugs before admission may be one cause of decreases in the serum AGR, such as corticosteroids, which are routinely used in elderly patients with COPD, previous studies confirmed that long-term use of corticosteroids was associated with the occurrence of hypoalbuminemia (16).
Infection is one of the main causes of AECOPD and increased mortality (17). Traditional microorganism culture has the disadvantages of needing a long time to complete and having low sensitivity. Therefore, it would be beneficial to guide clinical treatment and improve patients’ quality of life by finding a new, simple, and inexpensive testing indicator for quick determination of whether infection is present in patients with AECOPD. Age, PA level, and CRP level are strongly associated with the occurrence of infection (18,19). Our results also verified that age, PA level, CRP level, and AGR were significantly different between patients with and without infection. After grouping according to the optimal cutoff values of the PA level, CRP level, and AGR, we observed a difference in the rate of combined infection. However, the cutoff value of the AGR (1.37) had high sensitivity and specificity in determining the presence of infection in patients with AECOPD. An animal study verified that the AGR was obviously lower in cattle with severe infection than in healthy cattle (20). The AGR is also an effective marker for ruling out feline infectious peritonitis (21). Moreover, Salazar-Kagunye et al. (22) found that the AGR was noticeably reduced in patients with Clostridium difficile infection. Therefore, we believe that the AGR can be applied as a reference marker for determining the presence of infection in elderly patients with AECOPD.
The type, severity, and corresponding treatment methods of AECOPD are decisive factors that influence the hospital stay. A survey of hospitalized residents in southern Spain showed that the albumin concentration at admission was associated with the hospital stay (23). The present study confirmed that the AGR was negatively associated with the hospital stay, which was longer in patients with an AGR of <1.37 than ≥1.37. These findings suggested that in the early stage of admission, the AGR can be used to select the appropriate treatment intensity and establish a reasonable clinical pathway. Patients with a high AGR and less severe clinical condition can move to low-level hospital to avoid over-treatment, which can effectively shorten the hospitalization time in high-level hospitals and reduce the loss of economic and social benefits. The serum albumin concentration is a prognostic factor in many lung diseases (24,25). We did not follow up on the prognosis of our patients, but early studies have shown that the AGR can be utilized to evaluate tumor prognosis (26,27). The AGR was combined with two prognostic factors: the serum albumin and globulin concentrations. We hypothesized that use of the AGR for prognostic evaluation of AECOPD is superior to use of the serum albumin concentration.
This study has some limitations. First, this study only included the medical records of elderly patients with COPD in Nanning of the Guangxi Zhuang Autonomous Region of China, and the population representation was not strong. Second, changes in related indexes before and after treatment were not analyzed, and the patients were not followed up after discharge. Third, we did not consider various factors that may be related to the AGR, such as the patients’ treatment and medication history.
Conclusions
The results of this study provide evidence that use of the AGR is beneficial in the assessment of the clinical condition, presence of infection, treatment options, clinical path, and prognosis in elderly patients with AECOPD.
Acknowledgements
This work was supported by Department of Clinical Laboratory, The First Affiliated Hospital of Guangxi Medical University.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University, China (No. 2018-KY-E-020).
References
- Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000;117:398S-401S. [Crossref] [PubMed]
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Erratum to "Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary" Arch Bronconeumol 2017;53:411-2. [Arch Bronconeumol. 2017;53:128-49]. [Crossref] [PubMed]
- Shahriary A, Panahi Y, Shirali S, et al. Relationship of serum levels of interleukin 6, interleukin 8, and C-reactive protein with forced expiratory volume in first second in patients with mustard lung and chronic obstructive pulmonary diseases: systematic review and meta-analysis. Postepy Dermatol Alergol 2017;34:192-8. [Crossref] [PubMed]
- Deng Y, Pang Q, Miao RC, et al. Prognostic significance of pretreatment albumin/globulin ratio in patients with hepatocellular carcinoma. Onco Targets Ther 2016;9:5317-28. [Crossref] [PubMed]
- Liu J, Dai Y, Zhou F, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol Oncol 2016;34:484.e1-e8. [Crossref] [PubMed]
- Lee J, Machin M, Russell KE, et al. Corticosteroid modulation of immunoglobulin expression and B-cell function in COPD. FASEB J 2016;30:2014-26. [Crossref] [PubMed]
- Restrepo MI, Anzueto A. Guidelines for the diagnoses and treatment of adult lower respiratory tract infections: a true "European cooperative effort". Eur Respir J 2005;26:979-81. [Crossref] [PubMed]
- Azab B, Bibawy J, Harris K, et al. Value of Albumin-Globulin Ratio as a Predictor of All-Cause Mortality After Non-ST Elevation Myocardial Infarction. Angiology 2013;64:137-45. [Crossref] [PubMed]
- Pal GK, Shyma P, Habeebullah S, et al. Association of albumin-globulin ratio with sympathovagal imbalance in pregnancy-induced hypertension. Indian J Physiol Pharmacol 2011;55:128-38. [PubMed]
- Cornwell WD, Kim V, Song C, et al. Pathogenesis of inflammation and repair in advanced COPD. Semin Respir Crit Care Med 2010;31:257-66. [Crossref] [PubMed]
- Azab BN, Bhatt VR, Vonfrolio S, et al. Value of the pretreatment albumin to globulin ratio in predicting long-term mortality in breast cancer patients. Am J Surg 2013;206:764-70. [Crossref] [PubMed]
- Curtis JL, Freeman CM, Hogg JC. The immunopathogenesis of chronic obstructive pulmonary disease: insights from recent research. Proc Am Thorac Soc 2007;4:512-21. [Crossref] [PubMed]
- Feghali-Bostwick CA, Gadgil AS, Otterbein LE, et al. Autoantibodies in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:156-63. [Crossref] [PubMed]
- Incalzi RA, Corsonello A, Pedone C, et al. Chronic renal failure: a neglected comorbidity of COPD. Chest 2010;137:831-7. [Crossref] [PubMed]
- Chen CW, Chen YY, Lu CL, et al. Severe hypoalbuminemia is a strong independent risk factor for acute respiratory failure in COPD: a nationwide cohort study. Int J Chron Obstruct Pulmon Dis 2015;10:1147-54. [Crossref] [PubMed]
- Li XW, Jiang RM, Guo JZ. Glucocorticoid in the treatment of severe acute respiratory syndrome patients: a preliminary report. Zhonghua Nei Ke Za Zhi 2003;42:378-81. [PubMed]
- Lieberman D, Lieberman D, Ben-Yaakov M, et al. Infectious etiologies in acute exacerbation of COPD. Diagn Microbiol Infect Dis 2001;40:95-102. [Crossref] [PubMed]
- Emami Ardestani M, Zaerin O. Role of Serum Interleukin 6, Albumin and C-Reactive Protein in COPD Patients. Tanaffos 2015;14:134-40. [PubMed]
- Ning J, Shao X, Ma Y, et al. Valuable hematological indicators for the diagnosis and severity assessment of Chinese children with community-acquired pneumonia: Prealbumin. Medicine (Baltimore) 2016;95. [Crossref] [PubMed]
- Yurtseven S, Uysal H. Decreased serum sialic acid, albumin-globulin ratio and total protein levels in cattle heavily infected with Theileria annulata. Vet Fak Derg 2009;56:141-4.
- Jeffery U, Deitz K, Hostetter S. Positive predictive value of albumin: globulin ratio for feline infectious peritonitis in a mid-western referral hospital population. J Feline Med Surg 2012;14:903-5. [Crossref] [PubMed]
- Salazar-Kagunye R, Shah A, Loshkajian G, et al. Association of decreased serum protein fractions with Clostridium difficile infection in the acute care setting: a case-control study. Biomark Med 2012;6:663-9. [Crossref] [PubMed]
- Delgado-Rodríguez M, Marcelino-Cuadros M, Gomez-Ortega A, et al. Cholesterol and serum albumin levels as predictors of cross infection, death, and length of hospital stay. Arch Surg 2002;137:805-12. [Crossref] [PubMed]
- Lowes D, Al-Shair K, Newton PJ, et al. Predictors of mortality in chronic pulmonary aspergillosis. Eur Respir J 2017.49. [PubMed]
- Aman J, van der Heijden M, van Lingen A, et al. Plasma protein levels are markers of pulmonary vascular permeability and degree of lung injury in critically ill patients with or at risk for acute lung injury/acute respiratory distress syndrome. Crit Care Med 2011;39:89-97. [Crossref] [PubMed]
- He X, Guo S, Chen D, et al. Preoperative Albumin to Globulin Ratio (AGR) as Prognostic Factor in Renal Cell Carcinoma. J Cancer 2017;8:258-65. [Crossref] [PubMed]
- Zhang F, Sun P, Wang ZQ, et al. Low preoperative albumin-globulin score predicts favorable survival in esophageal squamous cell carcinoma. Oncotarget 2016;7:30550-60. [PubMed]


