Role of animal models for percutaneous atrial septal defect closure
Introduction
Transcatheter device occlusion of secundum atrial septal defects (ASD) has become the currently preferred treatment strategy while surgical closure is now dedicated to patients with unsuitable anatomic features or associated cardiac malformations (1,2). Since the first description of Drs. King and Mills in 1974, the great majority of percutaneous devices developed to close those defects rely on self-expandable occluders that enclose the defect in a sandwich-like fashion (3) At the time of this writing, the Amplatzer septal occluder (ASO) (Abbott, St. Jude Medical, St. Paul, MN, USA), is the most commonly used device due to its innovative design as well as its straightforward deployment technique, repositionability and retrievability (4). Similar to the ASO design, most devices are based on self-expandable double disk consisting of a nitinol wire mesh and a polymeric membrane to ensure a perfect sealing.
Regarding their design, most of the occluding disks are larger than the ASD’s diameter and attach to the cardiac surface in a sutureless fashion. However, device oversizing and stretching of the defect may in some cases not allow for an appropriate apposition of the device to the atrial endocardium. Therefore, it has been suggested that some long-term device related complications such as thrombosis or infective endocarditis may be associated with an inappropriate healing or endothelialisation of the ASD closure device (5). These observations led industrial companies which develop those ASD occluders to pay great attention on the healing process and to direct their development toward novel materials which are supposed to “enhance” or to “accelerate” device endothelialisation. As for any implantable device, large animal models of ASD have been widely used throughout the historical developments of ASD closure devices (3,6-20).
In this review we aimed to: (I) present the different types of animal models used in the setting of percutaneous ASD closure; (II) focus on the device endothelialisation process and the other issues analysed through these models and (III) evaluate the future developments which may improve the contribution of animal models for the understanding of endothelialisation and healing processes.
Which animal for which defect?
After completion of rigorous bench testing, the research and development process of percutaneous occluders generally proceeds with a large animal study. These experiments are performed for different aims but mostly for feasibility and safety assessment needed for premarket CE mark or FDA approval. Those models differ according to the type of species that are studied (porcine, ovine or canine model) and the way of creating the defect (percutaneously or surgically).
Regarding the choice of the species, the latter must have anatomical characteristics, concerning the inter-atrial septum, close to the human. In the case of sheep and pig models, these specifications appear to be well filled, whereas the canine model does not seem to have enough anatomical similarities with the human heart. Indeed, swine and ovine models may be used because of the well-developed fossa ovalis and comparable atrial septal anatomy to that of humans, allowing the creation of defects that closely mimic human secundum ASDs in respect of both size and location. On the contrary, the canine atrial anatomy widely differs from that of humans, especially due to the presence of a prominent crista terminalis in the right atrium while the septal surfaces are small on both atrial sides compared with the human heart (3,6,8,13,19). Thus, the canine model, mainly used in the early publications, has been virtually abandoned since the study of Sharafuddin et al. who used a pig model to assess the feasibility and safety of the ASO (10-12,14-18,20). One can imagine that the design of most of the occluders developed since then and resembling the ASO necessitated the presence of large septal surfaces which excluded the canine model. Of note, a sheep model has been used to evaluate the biocompatibility of the Nitinol alloy, which contributed greatly to developing ASD occlusion devices, especially the ASO, due to its low profile and its ability to reshape itself after catheter deployment (21). Nevertheless, whatever the type of canine, ovine or porcine model used, these species are also interesting because of their capacity for rapid growth, which can reproduce in a few months the growth of a child becoming an adult.
Once the animal species has been chosen, the researchers have to choose the approach from which the ASD would be created, either surgical or percutaneous. Surgical ASD is mostly performed through a transverse left thoracotomy and beating heart. No imaging guidance is required. Usually, clamps are placed across the left and right atrial appendages which are entered through purse-string sutures. A sharp punch instrument is then introduced from the left atrial appendage through the purse-string suture and, guided to the fossa ovalis with the opposing index finger, is passed from the left to the right atrium. The punch choice depends on the desired ASD size. After creation of the defect, the thoracotomy is closed, and the animal allowed to recover for several weeks before the transcatheter device closure (8,10,13,18).
Percutaneous ASD creation is achieved by inter-atrial trans-septal puncture, usually under fluoroscopic and either transthoracic, transoesophageal or intra-cardiac echography (7,9,11,12,14,15,17,19). After femoral veins cannulation, a transseptal sheath and Brockenbrough needle are placed into the superior vena cava and then onto the inter-atrial septum toward the fossa ovalis. Atrial septum is then punctured and a stiff wire is positioned into the left atrium or a pulmonary vein while heparin is administered. The ASD is then created by subsequent balloon dilation of the interatrial septum using balloons of the defect’s desired diameters (Figure 1). The ASD is either subsequently closed during the same procedure or after allowing for healing of the created defect edges for several weeks (14). Following percutaneous closure, the anti-thrombotic regimen widely varies between the studies, regardless of the approach used for ASD creation or the animal species, from absence treatment (10,14,15) to a dual anti-platelet regimen including aspirin and clopidogrel for 3 to 6 months (17). Some authors advocate both cost-effectiveness and a low level of evidence, especially in sheep (22), to support the absence of treatment.
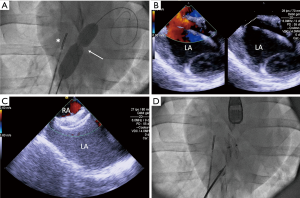
Apart from the obvious minimal invasiveness of the percutaneous approach, each technique used for ASD creation has its own advantages and drawbacks. On the one hand, surgically created ASD cannot duplicate the noncircular shape and variable position of secundum ASDs seen in humans while acute septal injury associated with the punch could have effect on the healing process after device implantation. On the other hand, almost same disadvantages may be attributed to the percutaneous technique, especially the fact that ASDs created by transseptal puncture and subsequent balloon dilation, lead to a fresh wound in the septal wall that could potentially alter the healing response to devices implanted thereafter. Finally, both techniques failed to create defect with deficient rims, especially the aortic one, which would be useful to test the performance of some dedicated devices.
Assessment of device endothelialisation
The primary aim of animal studies was to assess the feasibility and safety of newly developed devices for premarket approval. This goal was most of the time reached as those models offer the possibility of evaluating the deployment technique, repositionability and retrievability of occluders. Nonetheless, understanding the biocompatibility, the healing process and the endothelialisation of devices became with time a critical point of animal studies, as it has been suggested that some long-term device related complications may be associated with an inappropriate healing or endothelialisation of the ASD closure device (5,23-25). Among the most relevant available studies, the evaluation of endothelialisation was assessed after animal sacrifice and explantation which was performed after sequential duration follow-up ranging from 1 week to 2 years (Table 1). The work-up included macroscopic evaluation, histopathological study with optical microscopy and in some cases scanning electron microscopy (13,15,19). Neoendothelialisation was evaluated using routine staining (haematoxylin and eosin, toluidine blue or Richardson blue) as well as immunohistochemistry. Endothelial cells were observed as soon as 30 days after implantation and neo-endothelialisation seemed to be completed after 3 to 6 months of follow-up (Figure 2) (10,13,15,16). A chronic inflammatory response directed against textile fibres of devices was also frequently observed but without thrombus deposition and regardless of the antithrombotic regimen which was used (15). However, such results are, at best, only partly predictive as the human body is known to respond in a different fashion to other implantable devices than do canine, ovine or porcine species (26).

Full table
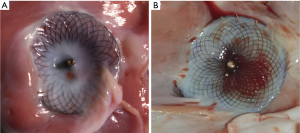
Combining this with the above-mentioned limitations of ASD creation models, the transposition of animal models results to humans seems debatable and need some validating comparison with human healing process. Indeed, excluding isolated reports, a systematic evaluation of the healing response to ASD occluders in humans has rarely been performed. This is mostly due to the fact that this assessment can only be performed on explanted devices. Some authors have also tried to compare the occluders healing and endothelialisation in humans to that observed in animal models, in order to validate these experimental findings.
Kreutzer et al. examined the healing responses to the clamshell device in occluders explanted at least 1 month following implantation, in order to compare the histopathologic findings with previously observed results from experimental implantation in lambs (7) and in canine models (27,28). Twelve explanted devices were examined, after a median delay from implantation of 1.6 years. Gross examination revealed that a majority of devices were completely or almost completely covered by a white, non-thrombotic glistening pseudointima of variable thickness. Histologically, it corresponded to fibro-elastic pseudointima including dense fibrous tissue with predominance of collagen. Focal foreign body reaction with giant polynucleated cells was typically observed at the interface with the fabric. The rarely observed thrombi were located in the atrial wall opposite to a device fracture. Authors concluded that the healing response in humans for that device was not significantly different from that observed in animal models.
A decade later, Sigler et al. performed similar studies by comparing the healing of the ASD occlusion devices in two series of human and animal experimental explants within the same protocol using a uniform histopathological work up (15,29). In their most recent work, which focuses on ASO and Starflex devices, the devices implant ranged from 5 days to 48 months in humans and 4 days to 12 months in sheep. Within the first days after implantation, fibrin condensation and accumulation of thrombotic material was seen on the devices while cellular organisation was shown to proceed in the initial months after implant. Scanning electron microscopy performed on both human and animal specimens with a follow-up period of >90 days showed a complete neoendothelial coverage of the protruding metal framework without thrombus. Moreover, the authors confirmed the presence of a mild chronic inflammatory response directed against textile fibres of the devices equally in human and animal samples. Another paper published by the same team further investigated this field by studying cellular and extracellular matrix components that are formed within and at the surface of human explanted occluders in order to identify antigen characteristics of neotissues (30). One of the main findings of their work, conducted on Cardioseal/Starflex and the ASO devices explanted between 5 days and 48 months after initial procedures, was to observe a functional neoendothelium in all specimens with implantation times >10 weeks.
Based on these different works the authors concluded that (I) Tissue reactions in experimental animals adequately reproduce the healing response to ASD occlusion devices in humans especially regarding neo-endothelialisation and (II) the timeline of the healing process further supports the clinical guidelines of antithrombotic therapy for 6 months after implantation.
However, due to several limitations, one should be cautious when drawing conclusions from retrospective histopathological studies in devices implanted in humans, as it is the case for animal models. First, these studies are performed on very small series of human explants in which the healing response may not be representative of that observed in successfully implanted occluders. Second, the timeline of specimen analysis is arbitrarily determined by the date of explant precluding a sequential evaluation at predetermined post-procedural periods. Third, as the great majority of studied devices were surgically removed, possible additional trauma due to device manipulation may occur during the explant. In addition, two well-recognized and possibly delayed device-related complications are associated with an incomplete healing of ASD occluders: device-related thrombosis and infective endocarditis (5). Indeed, cases of incomplete neoendothelialisation from 18 months up to 7 years after device implantation have been reported with the ASO (31,32).
Therefore, there is an undetermined proportion of cases of delayed/incomplete endothelialisation of devices, and to date, there is no specific method for confirming complete endothelialisation on the occluder surface in individual patients. Thus, if more than 6 months is necessary for complete neoendocardial coverage of the device, the risk of thrombus formation and infective endocarditis may persist after the recommended duration of anti-platelet therapy and infective endocarditis prophylaxis (33). These observations confirm that some caution is required when transposing results derived from animal models to routine clinical practice.
Other issues?
Other than device endothelialisation, animal models of percutaneous ASD closure focused on other complications associated with this technique including nickel hypersensitivity. Indeed, after percutaneous ASD closure, concerns have been raised about the potential release and hypersensitivity reactions to nickel, especially with the ASO (5,34). In patients implanted with the ASO, symptoms associated with nickel hypersensitivity have been correlated to nickel levels in blood samples, as well as in in-vitro studies (35,36).
Therefore, when developing new devices, researchers and companies use the decreased or low nickel release—due to a modified design or a specific nitinol mesh coating—as potential marketing tools. These experiments included sequential measurements (before implantation and up to 12 months after the procedure of (I) The nickel concentration of the whole blood assessing systemic release and (II) nickel concentration of atrial samples in close proximity to the device assessing tissular release. The Helex device experiments showed no statistical difference between test and control samples taken at any time interval up to the 12 months post-procedure (13). The CeraFlex occluder showed a nickel blood content increase by a factor of three compared to the level before operation and a decrease afterwards returning to the normal level after six months when endothelialisation was complete (16). Nevertheless, no comparative in vivo nickel release assays between a newly designed device and the ASO have been conducted to the best of our knowledge.
Other authors evaluated the short-term effects of right ventricular (RV) volume overload on RV contractility using a swine model of percutaneously created ASD (37). The left-to-right atrial shunt was created percutaneously, either by balloon dilatation of the fossa ovalis, by implantation of a multi-perforated ASO or a patch-less nitinol device. After a follow-up of 4.6 weeks, the authors concluded that this period of chronic RV volume overload does not alter RV contractility significantly. Other interesting studies involving ASD animal models included the assessment and closure of small shunts with magnetic resonance guidance in a swine model (38,39). Despite early promising results, this technique has not been further developed, possibly due to concerns about the MR safety of guidewires and device delivery systems. Finally, no animal model focused on one of the most concerning complication following percutaneous ASD closure which is cardiac erosion (40).
What’s next?
Owing to the partially predictive results of endothelialisation assessment in animal models, as the human heart is known to respond in a different fashion to canine, ovine or porcine species (26) one solution might be studying the endothelialisation process using human endothelial cells through in-vitro experiments. Such studies focusing on ASD closure devices are scarce, due to the difficulties to recreate in-vitro the complete process of endothelialisation. Using human endothelial progenitor cells, Kong et al. demonstrated that Nitinol showed satisfactory biocompatibility which can be improved by coating with recombinant hirudin (rHirudin) or fibronectin with regard to anti-thrombogenicity and endothelialisation (41). However, the authors did not perform the tests on commercially available devices. Nonetheless, although these in-vitro models also have limitations, especially due to the absence of element of blood, plasma proteins, complement system or shear stress, they might be complementary with in-vivo animal models.
Another research direction is to develop tool for an individual assessment of device endothelialisation. As previously mentioned, there is an undetermined proportion of cases of delayed/incomplete device endothelialisation and, to date, no specific method to confirm it in individual patients (31,32).
Some authors developed a few years ago a self-actuating, self-sensing device for detecting the presence of endothelial cells on a surface of a coronary stent in order to detect when the struts have been covered with a layer of endothelial cells (42). The aim of this in-vitro study was to develop a tool that would allow for an anti-platelet therapy adaptation in real-time with regards to the patient’s level of healing. So far, no clinical application of this method has been published but one can imagine that the translation of this technology to intracardiac occluder might be promising. A recent clinical study comparing the biological markers of inflammation and proliferation between 3 commercially available devices (ASO, Lifetech CeraFlex, or Occlutech Figulla Flex II) following percutaneous closure in children also showed promising results although the follow-up period was too short (1 month) (43).
Finally, the development of imaging techniques that could non-invasively assess the complete endothelialisation of a device might be helpful. High-resolution MRI technologies or ultra-fast echo techniques (44,45), which showed encouraging results in similar cardiologic fields might constitute exciting new research directions in a near future.
Conclusions
Large animal models of ASD have been widely used throughout the historical developments of ASD closure devices. Other than the feasibility and safety of newly developed devices, those experiments mostly aim to study the healing process and endothelialisation of devices, as some long-term device related complications may be associated with an inappropriate endothelialisation of the occluder. Tissue reactions in experimental models were shown to adequately reproduce the healing response to ASD occlusion devices in humans, with an endothelial device coverage observed as soon as 30 days after implantation and complete after 3 to 6 months. However, there is an undetermined proportion of cases of delayed/incomplete device endothelialisation and future research directions may focus on developing specific methods for confirming complete endothelialisation on the occluder surface in individual patients.
Acknowledgements
Funding: This study received financial support from the French Government as part of the “Investments of the future” program managed by the National Research Agency (ANR), Grant reference ANR-10-IAHU-04.
Footnote
Conflicts of Interest: Z Jalal and AE Baruteau received a research grant from Saint-Jude Medical. The other authors have no conflicts of interest to declare.
References
- Du ZD, Hijazi ZM, Kleinman CS, et al. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multi- center nonrandomized trial. J Am Coll Cardiol 2002;39:1836-44. [Crossref] [PubMed]
- Mylotte D, Quenneville SP, Kotowycz MA, et al. Long-term cost- effectiveness of transcatheter versus surgical closure of secundum atrial septal defect in adults. Int J Cardiol 2014;172:109-14. [Crossref] [PubMed]
- King TD, Mills NL. Nonoperative closure of atrial septal defects. Surgery 1974;75:383-8. [PubMed]
- Bissessor N. Current perspectives in percutaneous atrial septal defect closure devices. Med Devices (Auckl) 2015;8:297-303. [Crossref] [PubMed]
- Jalal Z, Hascoet S, Baruteau AE, et al. Long-term Complications After Transcatheter Atrial Septal Defect Closure: A Review of the Medical Literature. Can J Cardiol 2016;32:1315.e11-1315.e18. [Crossref] [PubMed]
- Rashkind WJ, Cuaso CE. Transcatheter closure of atrial septal defects in children. Eur J Cardiol 1977;8:119-20.
- Lock JE, Rome JJ, Davis R, et al. Transcatheter Closure of Atrial Septal Defects Experimental Studies. Circulation 1989;79:1091-9. [Crossref] [PubMed]
- Das GS, Voss G, Jarvis G, et al. Experimental atrial septal defect closure with a new, transcatheter, self-centering device. Circulation 1993;88:1754-64. [Crossref] [PubMed]
- Pavcnik D, Wright KC, Wallace S. Monodisk: device for percutaneous transcatheter closure of cardiac septal defects. Cardiovasc Intervent Radiol 1993;16:308-12. [Crossref] [PubMed]
- Sharafuddin MJ, Gu X, Titus JL, et al. Transvenous closure of secundum atrial septal defects: preliminary results with a new self-expanding nitinol prosthesis in a swine model. Circulation 1997;95:2162-8. [Crossref] [PubMed]
- Bloch Thomsen A, Schneider M, Baandrup U, et al. Animal experimental implantation of an atrial septal defect occluder system. Heart 1998;80:606-11. [Crossref] [PubMed]
- Sideris EB, Kaneva A, Sideris SE, et al. Transcatheter atrial septal defect occlusion in piglets by balloon detachable devices. Catheter Cardiovasc Interv 2000;51:529-34. [Crossref] [PubMed]
- Zahn EM, Wilson N, Cutright W, et al. Development and Testing of the Helex Septal Occluder, a New Expanded Polytetrafluoroethylene Atrial Septal Defect Occlusion System. Circulation 2001;104:711-6. [Crossref] [PubMed]
- Jux C, Bertram H, Wohlsein P, et al. Interventional atrial septal defect closure using a totally bioresorbable occluder matrix: development and preclinical evaluation of the BioSTAR device. J Am Coll Cardiol 2006;48:161-9. [Crossref] [PubMed]
- Sigler M, Jux C. Biocompatibility of septal defect closure devices. Heart 2007;93:444-9. [Crossref] [PubMed]
- Zhang D, Zhang Z, Zi Z, et al. Fabrication of graded TiN coatings on nitinol occluders and effects on in vivo nickel release. Biomed Mater Eng 2008;18:387-93. [PubMed]
- Krizanic F, Sigler M, Figulla HR. Transvenous Closure of Patent Foramen Ovale: Preliminary Results with a New Self Expanding Nitinol Wire Mesh in a Swine Model. Cardiol Res Pract 2009;2009. [Crossref] [PubMed]
- Wu W, Yip J, Tang YD, et al. A novel biodegradable septal defect occluder: the "Chinese Lantern" design, proof of concept. Innovations (Phila) 2011;6:221-30. [Crossref] [PubMed]
- Zhu YF, Huang XM, Cao J. Animal experimental study of the fully biodegradable atrial septal defect (ASD) occluder. J Biomed Biotechnol 2012;2012. [Crossref] [PubMed]
- Sigler M, Söderberg B, Schmitt B, et al. Carag bioresorbable septal occluder (CBSO): histopathology of experimental implants. EuroIntervention 2018;13:1655-61. [Crossref] [PubMed]
- Cragg AH, De Jong SC, Barnhart WH, et al. Nitinol intravascular stent: results of preclinical evaluation. Radiology 1993;189:775-8. [Crossref] [PubMed]
- Spanos HG. Aspirin fails to inhibit platelet aggregation in sheep. Thromb Res 1993;72:175-82. [Crossref] [PubMed]
- Moore J, Hegde S, El-Said H, et al. Transcatheter device closure of atrial septal defects: a safety review. JACC Cardiovasc Interv 2013;6:433-42. [Crossref] [PubMed]
- Kim DJ, Shim CY, You SC, et al. Late Bacterial Endocarditis and Abscess Formation After Implantation of an Amplatzer Septal Occluder Device. Circulation 2015;131:e536-8. [Crossref] [PubMed]
- Krumsdorf U, Ostermayer S, Billinger K, et al. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol 2004;43:302-9. [Crossref] [PubMed]
- Ortenwall P, Bylock A, Kjellstrom BT, et al. Seeding of ePTFE carotid interposition grafts in sheep and dogs: species-dependent results. Surgery 1988;103:199-205. [PubMed]
- Kuhn MA, Latson LA, Cheatham JP, et al. Biological response to Bard Clamshell septal occluders in the canine heart. Circulation 1996;93:1459-63. [Crossref] [PubMed]
- Kreutzer J, Ryan CA, Gauvreau K, et al. Healing response to the Clamshell device for closure of intracardiac defects in humans. Catheter Cardiovasc Interv 2001;54:101-11. [Crossref] [PubMed]
- Sigler M, Paul T, Grabitz RG. Biocompatibility screening in cardiovascular implants. Z Kardiol 2005;94:383-91. [Crossref] [PubMed]
- Foth R, Quentin T, Michel-Behnke I, et al. Immunohistochemical characterization of neotissues and tissue reactions to septal defect-occlusion devices. Circ Cardiovasc Interv 2009;2:90-6. [Crossref] [PubMed]
- Chessa M, Butera G, Frigiola A, et al. Endothelialization of ASD devices for transcatheter closure: possibility or reality? Int J Cardiol 2004;97:563-4. [Crossref] [PubMed]
- Chen F, Zhao X, Zheng X, et al. Incomplete endothelialization and late dislocation after implantation of an Amplatzer septal occluder device. Circulation 2011;124:e188-9. [Crossref] [PubMed]
- Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57. [Crossref] [PubMed]
- Rodés-Cabau J, Mineau S, Marrero A, et al. Incidence, timing, and predictive factors of new-onset migraine headache attack after transcatheter closure of atrial septal defect or patent foramen ovale. Am J Cardiol 2008;101:688-92. [Crossref] [PubMed]
- Ries MW, Kampmann C, Rupprecht HJ, et al. Nickel release after implantation of the Amplatzer occluder. Am Heart J 2003;145:737-41. [Crossref] [PubMed]
- Verma DR, Khan MF, Tandar A, et al. Nickel elution properties of contemporary interatrial shunt closure devices. J Invasive Cardiol 2015;27:99-104. [PubMed]
- Uebing A, Fischer G, Schlangen J, et al. Interventional creation of an atrial septal defect and its impact on right ventricular function: an animal study with the pressure-volume conductance system. Cardiol J 2011;18:289-96. [PubMed]
- Rickers C, Jerosch-Herold M, Hu X, et al. Magnetic resonance image-guided transcatheter closure of atrial septal defects. Circulation 2003;107:132-8. [Crossref] [PubMed]
- Schalla S, Saeed M, Higgins CB, et al. Balloon sizing and transcatheter closure of acute atrial septal defects guided by magnetic resonance fluoroscopy: assessment and validation in a large animal model. J Magn Reson Imaging 2005;21:204-11. [Crossref] [PubMed]
- Diab K, Kenny D, Hijazi ZM. Erosions, erosions, and erosions! Device closure of atrial septal defects: how safe is safe? Catheter Cardiovasc Interv 2012;80:168-74. [Crossref] [PubMed]
- Kong X, Grabitz RG, van Oeveren W, et al. Effect of biologically active coating on biocompatibility of Nitinol devices designed for the closure of intra-atrial communications. Biomaterials 2002;23:1775-83. [Crossref] [PubMed]
- Musick KM, Coffey AC, Irazoqui PP. Sensor to detect endothelialization on an active coronary stent. Biomed Eng Online 2010;9:67. [Crossref] [PubMed]
- Aydın Şahin D, Başpınar O, Sülü A, et al. A comparison of the in vivo neoendothelialization and wound healing processes of three atrial septal defect occluders used during childhood in a nonrandomized prospective trial. Anatol J Cardiol 2017;18:229-34. [PubMed]
- Maresca D, Correia M, Villemain O, et al. Noninvasive Imaging of the Coronary Vasculature Using Ultrafast Ultrasound. JACC Cardiovasc Imaging 2018;11:798-808. [Crossref] [PubMed]
- Vaillant F, Magat J, Bour P, et al. Magnetic resonance-compatible model of isolated working heart from large animal for multimodal assessment of cardiac function, electrophysiology, and metabolism. Am J Physiol Heart Circ Physiol 2016;310:H1371-80. [Crossref] [PubMed]


