The role of a multidisciplinary severe chronic obstructive pulmonary disease hyperinflation service in patient selection for lung volume reduction
Introduction
Chronic obstructive pulmonary disease (COPD) is a highly prevalent disease and currently the third leading cause of death worldwide after heart disease and cancer (1). The characteristic airflow obstruction is caused by a variety of pathological process such as small airway remodelling or loss of alveolar tissue but is usually a combination of both. Emphysema is a subtype of COPD characterised by permanent enlargement of the airspaces distal to the terminal bronchioles due to destruction of the alveolar walls. The resulting loss of gas exchange surface area, lung elastic recoil and expiratory airflow limitation leads to air trapping and hyperinflation of the lung. Emphysematous hyperinflation is thought to contribute to breathlessness through a variety of mechanisms including dynamic hyperinflation, impairment of diaphragmatic function, ventilation-perfusion mismatch, adverse nutritional effects and accelerated sarcopenia (1,2). In advanced cases, it severely limits quality of life due to intractable breathlessness, progressive disability, increased risk of mortality and eventually premature death.
Conservative management such as bronchodilator inhaler therapy, smoking cessation, pulmonary rehabilitation and long-term oxygen therapy have only demonstrated at best a modest benefit to symptoms. Most patients continue to decline with exacerbations, worsening exercise tolerance and progressive loss of function. Individuals can be increasingly reliant on assisted care and often experience loss of earnings due to unemployment. In response to this, there is the search for additional therapeutic interventions is order to target this high-risk group of patients with debilitating symptoms.
The concept of lung volume reduction surgery (LVRS) was originally formulated in the 1950s (3). The surgery is aimed at removing the diseased hyperinflated and functionless emphysematous lung, enhancing the function of the remaining, healthier lung tissue and diaphragm, which then leads to, improved lung and chest wall mechanics— ultimately improving breathlessness and exercise tolerance. Experiences with LVRS in the 1990s were inconsistent with variable outcomes and a high morbidity and mortality until the publication of the National Emphysema Treatment Trial (NETT) in 2003. In this landmark study, a subgroup of patients with predominantly upper lobe emphysema, heterogenous disease and low exercise capacity demonstrated clinically significant improvement to exercise tolerance, quality of life and survival when treated with bilateral LVRS (4). In contrast, patients with low forced expiratory volume in one second (FEV1) of less than 20% and either homogenous emphysema or a very low transfer factor for carbon monoxide (TLCO) of less than 20% are at highest risk of death after surgery and are unlikely to benefit from bilateral LVRS (5). This strongly suggests the need to select patients carefully in order to achieve good clinical outcomes with these techniques (6).
Despite the potential life changing benefits of LVRS, the stigma associated with excess morbidity and mortality made surgery unpopular with only a low number of procedures performed. In the UK, only 164 LVRS procedures were undertaken between 2014 and 2015 (7). Over the past decade however, several non-surgical techniques to achieve LVR have been introduced. These include bronchoscopically inserted endobronchial valves (EBV), nitinol coils, thermal vapour and polymer sealant (8). Current research is most active in EBV treatment with supporting evidence of its use (9-11). In the STELVIO trial, in addition to well-documented improvement in FEV1, 79% of treated patients demonstrated a minimal clinically important difference in outcome markers representing quality of life and exercise tolerance as compared to only 33% in the control group (9). Pneumothorax is a common complication and the risks and benefits will be covered elsewhere in this journal.
Operative techniques and peri-operative management for LVRS have also improved over the years to adopt a less invasive unilateral and/or staged bilateral video assisted thoracoscopic (VATS) approach rather than the conventional bilateral midline sternotomy/open surgery. Audits of current practice have suggested that mortality and morbidity are significantly lower than previously described (12). The ongoing developments in bronchoscopic LVR techniques and surgical, anaesthetic and post-operative management of LVRS have emphasized the importance of patient selection as a key factor in improving outcomes. The nature of this process encourages the formation of specialist multidisciplinary teams (MDTs) to address the requirements of patient selection and matching to the most appropriate LVR intervention (13-15).
Provision of MDT
A MDT is a group of healthcare professionals with expertise in different fields, united as a team for the purpose of planning and implanting treatment programs for complex medical conditions (16). The concept of MDT gained prominence following the Calman-Hine report in 1995 specifically targeted for cancer care (17). Since then, MDT management for many medical conditions have taken a prominent role in patient management in many hospitals especially in resource rich countries. MDT management arguably has resulted in better care and improved survival however, the running of MDTs needs to be effective and coordinated with a clear leadership and core members buying in to the concept and their role in the MDT. Crucially, the MDT ethos should be one of open and constructive discussions in ensuring the best strategy is chosen for each individual patient.
COPD is a complex disease and in those with severe emphysema where LVR is being considered, specialist assessment with careful appraisal of risk/benefits is critical to good outcomes. In order to tailor the appropriate LVR intervention for patients, a MDT approach with expertise in managing emphysema is recommended by the National Institute of Clinical Excellence (NICE) (15).
However, the current provision of a structured advanced COPD service with access to LVR remains patchy and is not standardised across the UK. A previous British Thoracic Society survey report of attitudes of healthcare professionals to LVR found that most respiratory physicians were unsure about the mortality risk and significantly overestimate the risk of LVRS (18,19). This results in a “postcode lottery” as significant proportion of respiratory physicians reported having limited access to advanced COPD multidisciplinary services and LVR. This finding is also supported by a patient-centred study that reported patients having to fight for tertiary specialist referral and disappointment in the poor knowledge by healthcare professionals (20). It is not uncommon for patients to have to research these treatments themselves, and there is a general lack of knowledge and understanding of outcomes and pathways across the healthcare system (21). It is surprising that despite the high prevalence and healthcare burden of COPD, the establishment of MDTs are not as well organised compared to other diseases such as lung cancer. In this paper, we share our experiences of setting up and running a successful multidisciplinary severe COPD hyperinflation service, including our referral pathways and approach to patient selection for LVR procedures.
Structure and function of MDT
In Cambridge, we have been running a COPD hyperinflation MDT service since 2010 that are based at Addenbrooke’s and Royal Papworth Hospitals. Prior to the establishment of the MDT only a few cases of LVR were performed each year and there was no formal assessment pathway. The main aim of our MDT is to assess and offer treatments for emphysema to improve quality of life for COPD individuals. Our MDT is termed the hyperinflation MDT and we are aware that other providers have termed their MDT as COPD or Emphysema MDT. The structure of the MDT will vary according to local expertise and practice. The service provides access to LVR for a population of 5.8 million within East Anglia that is served by primary care practices, community respiratory nurse specialists and 13 acute trusts. In addition, we frequently receive referrals from outside of our region from centres that are 100–200 miles away. Over the years, our service has evolved to include more than one individual representing each specialty and improved cohesive pathways. Furthermore, it has expanded to cater for the growing number of referrals received and also refined for on-going service improvement. Critical to our development has been good working relationships and an annual half day meeting to review all elements of the pathway, to provide governance, review all deaths and to develop fresh ideas.
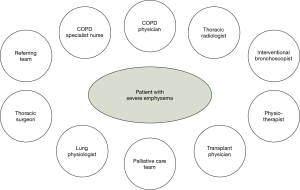
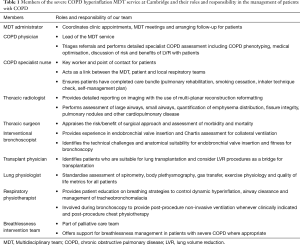
Our MDT is led by a COPD physician (we are aware that other MDTs have other specialities as leads) and includes core members—administrator, COPD specialist nurse, thoracic radiologist, thoracic surgeon, interventional bronchoscopist and respiratory physiotherapist. The wider MDT also includes other members including a transplant physician, breathlessness intervention service (part of the palliative care team) and respiratory physiologist (Figure 1). The roles and responsibilities of each member group in our MDT is summarised in Table 1. Each member of the MDT has a clearly delineated set of roles and responsibilities in order to work both individually and collaboratively in achieving a common goal of delivering personalised care for patients.
Full table
Our MDT meeting initially takes place on a bi-monthly basis adjusted annually to cope with demand. The MDT objective is to provide an in depth discussion of up to 10 patients in 90 minutes. Our assessment involves a multi-stage preliminary triage of patients to ensure that only the most promising cases are discussed at meetings, rather than discussing every referral. To ensure best use of time, only patients seen by a core member are discussed. Regular education during regional meetings with providers (primary care and secondary care specialist nurses, physiotherapists, secondary care physicians and thoracic surgeons) in the region has helped disseminate our criteria for referral. These referral criteria and referral pathway for patients being referred for LVR are illustrated in Figure 2.
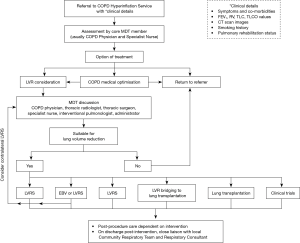
In a service evaluation carried out in 2016, we found that 67% of our patients referred to the service are discussed at the MDT meeting and approximately 40% of these were offered a treatment (22). The MDT meeting provides a forum for high quality case-specific discussion to appraise the risk and benefits to individual patients and to tailor the risk management pre- and post-procedure to reduce morbidity. All members of the MDT contribute to this and the main discussion points includes:
- anatomical and physiological suitability for the procedure;
- potential benefit of procedure and to determine whether unilateral VATs or EBV insertion is appropriate (the only two NICE approved therapies currently);
- predictable complications (especially risk and impact risk of pneumothorax post-EBV insertion) as well as establishing the risk of co-morbidities pose in limiting the benefits or increasing the risk of procedure;
- assessment of pulmonary reserve and overall risk especially vascular and cardiac;
- identifying target lobes, i.e., lobes with the highest emphysema destruction and poor function as determined by perfusion scan;
- optimisation of fitness before bronchoscopy or surgery and perioperative management.
Once the intervention has been decided, further coordination amongst MDT members regarding pre-operative medical optimisation and post-procedure care is orchestrated through standardised referral proforma’s coordinated through the service specific administrator and lead specialist nurse. Patients who may be suitable for staged bilateral LVR are identified from the initial MDT outcome to be re-discussed in the MDT meeting in due course.
Patient selection
Clinical assessment
As a starting point, any patient with severe emphysema who remains breathless despite achieving smoking cessation, on maximal inhaler therapy and who has undergone pulmonary rehabilitation should be referred for further assessment. The selection criteria for LVR should largely be based on clinical factors, degree of hyperinflation, patient motivation and goals. Assessment of collateral ventilation, emphysema pattern and distribution, physiological parameters and balancing the risk/benefit of procedure should also be considered. The inclusion and exclusion criteria for LVR are summarised in Table 2.

Full table
As COPD is a complex and heterogeneous disease, there is a wide spectrum of clinical patterns encompassed within the spectrum of severe COPD with severe airflow obstruction. In our service, the COPD physicians experienced in LVR and COPD therapies and prognosis performs the majority of clinical assessment of patients. The initial clinical assessment is evaluating the referring letter containing the patient’s clinical symptoms, co-morbidities, lung function test and computed tomography (CT) scan. All patients are offered a subsequent clinic review for a combined assessment by the COPD physician, specialist nurse and physiotherapist. The clinical assessment includes clinical phenotyping and establishing the relationship of emphysematous hyperinflation to clinical symptoms such as exacerbations and breathlessness. An understanding of the relative contribution of co-morbidities to symptom burden is equally important and patients with a reduced respiratory reserve often have comorbidities such as cardiac/vascular diseases and skeletal muscle deconditioning leading to an increased risk of mortality. In many cases, patient optimisation can occur following this consultation has identified other causes contributing to the patients symptoms or new pathology e.g., immunodeficiency, colonisation with resistant organism, tracheobronchomalacia, pulmonary hypertension, sleep apnoea and severe alpha-1-antitrypsin deficiency.
The patient’s expectations and concerns about treatment and goals are also explored and discussed. It is essential that the patient be clearly informed about the realistic outcomes relevant to their quality of life of the procedures and is counselled on the potential risks of peri-procedural complications. The specialist nurse provides further patient education, perform a care bundle checklist, provide relevant contact details and performs a capillary blood gas if appropriate. If deemed suitable for potential LVR procedures, further investigations are subsequently arranged.
Physiological measures
Determining suitability for LVR procedures will depend on accurate physiological assessment. A multi-modal approach should include measurement of different lung volumes, exercise capacity and health-related questionnaires. Assessment of static lung volumes via whole body plethysmography provides a multitude of measures, including the total lung capacity (TLC), residual volume (TV), inspiratory capacity and functional residual capacity. Hyperinflation and air trapping, which is a major driver of symptomatic limitations in COPD can be measured using the ratio of RV/TLC.
Is it recommended that all measurements throughout the LVR pathway should be gained through the same method. Our practice is to test all patients at our centre using whole body plethysmography to maintain consistency. Helium-dilution has been shown to underestimate TLC compared to plethysmographic values. Currently, this is a source for open debate as both methods are open to inaccuracies in severely obstructed patients with FEV1 <30% predicted. One benefit of whole body plethysmography is that airway resistance can be measured during testing. TLCO measurements are also important to understand the level of lung parenchymal destruction and the severity of disease and the recommended method of measurement is through body plethysmography. A TLCO value of below 20% predicted is a contraindication to LVR treatment due to the associated higher risk of mortality and morbidity following LVRS as demonstrated in the NETT trial (4).
Apart from lung function testing, our practice also includes assessment of the effects of respiratory symptoms and quality of life using standardized questionnaires such as the St. George’s Respiratory Questionnaire and the modified Medical Research Council dyspnoea scale. Exercise capacity is routinely measured using the 6-minute walk test as an alternative to more formal cardiopulmonary testing. This test is a validated, safe and easily reproducible with the distance covered in 6 minutes being the surrogate measure for exercise capacity (23). The overall assessments allow calculation of the BODE index (body-mass, airflow obstruction, dyspnoea, exercise) to facilitate discussion on prognosis with the patient.
Radiological assessment and collateral ventilation
Non-contrast high resolution CT on a multi-detector scanner platform with a thin series can provide useful assessment in LVR assessment. CTs are used to distinguish between emphysema predominant and airway predominant COPD (24). CTs can also characterise the type of emphysema (pan-acinar, centrilobular, paraseptal), degree of emphysema on a lobar basis, distribution of emphysema destruction (upper vs. lower), and to determine the integrity of lobar fissures. Further assessment of the pulmonary vasculature, the presence of lung nodules, bronchiectasis, lung cancer, interstitial fibrosis and severe tracheobronchomalacia are also useful in contributing to the eventual decision regarding LVR. We also perform 2-dimensional lung perfusion scans in order to assess the pulmonary circulation and decide the most appropriate target lobes.
The effectiveness of EBV treatment is not only dependent on optimal patient selection, but also correct placement of the valve in the target lobe. In addition, the absence of collateral ventilation is an important factor for successful lobar atelectasis and shrinkage. At our centre, we subjectively assess fissure integrity by using multi-planar reconstruction reformatting and categorise fissures into complete or incomplete defects with subcategories of minor (<10%) and major defects (>10%) (24). If the fissure is thought to be intact or a minor defect, the patient is potentially a suitable candidate for EBVs and bronchoscopic assessment of collateral ventilation is carried out. Chartis assessment using a balloon catheter to occlude the target bronchus and measurement of flow and resistance is performed. The added benefit of Chartis assessment is simultaneous bronchoscopic assessment and assessing the bronchial anatomy and feasibility of valve insertion, secretions and the extent of tracheobronchomalacia. In some institutions, assessment of collateral ventilation is aided by specific software analysis.
Conclusions
Lung volume reduction for emphysema is a potential life changing treatment for individuals with COPD. Patients are potentially high risk and patient selection and a multidisciplinary approach are critical to good outcomes and ongoing service development. The development of our multidisciplinary approach has transformed our ability to offer different modalities of treatment and so provide a proportion of our patients with a significant improvement in quality of life. We strongly recommend this approach.
Acknowledgements
All past and current members of the Cambridge Hyperinflation MDT.
Footnote
Conflicts of Interest: Outside of this work between 2013–2018 R Mahadeva discloses payment to attend meetings and for educational presentations/advisory boards on COPD from Kamada, Boehringer-Ingelheim, Pfizer, Chiesi, Astra-Zeneca, Pulmonx and grants for research from Grifols, Talecris and Pfizer Open Air. J Chew has no conflicts of interest to declare.
References
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease - 2018 Report [Internet]. Global Initiative for Chronic Obstructive Lung Disease; 2018. Available online: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf
- Brusasco V, Martinez F. Chronic obstructive pulmonary disease. Compr Physiol 2014;4:1-31. [PubMed]
- Brantigan OC, Mueller E. Surgical treatment of pulmonary emphysema. Am Surg 1957;23:789-804. [PubMed]
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [Crossref] [PubMed]
- National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075-83. [Crossref] [PubMed]
- Criner GJ, Cordova F, Sternberg AL, et al. The National Emphysema Treatment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med 2011;184:881-93. [Crossref] [PubMed]
- Society for Cardiothoracic Surgery in Great Britain and Northern Ireland. Outcomes: Thoracic. [Internet]. Available online: http://scts.org/outcomes/thoracic/
- Shah PL, Herth FJ, van Geffen WH, et al. Lung volume reduction for emphysema. Lancet Respir Med 2017;5:147-56. [Crossref] [PubMed]
- Klooster K, ten Hacken NHT, Hartman JE, et al. Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation. N Engl J Med 2015;373:2325-35. [Crossref] [PubMed]
- Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet 2015;386:1066-73. [Crossref] [PubMed]
- Kemp SV, Slebos DJ, Kirk A, et al. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (TRANSFORM). Am J Respir Crit Care Med 2017;196:1535-43. [Crossref] [PubMed]
- Clark SJ, Zoumot Z, Bamsey O, et al. Surgical approaches for lung volume reduction in emphysema. Clin Med (Lond) 2014;14:122-7. [Crossref] [PubMed]
- Rathinam S, Oey I, Steiner M, et al. The role of the emphysema multidisciplinary team in a successful lung volume reduction surgery programme†. Eur Assoc Cardio-Thorac Surg 2014;46:1021-6; discussion 1026.
- Lung volume reduction surgery for advanced emphysema. [Internet]. National Institute for Health and Care Excellence; Available online: https://www.nice.org.uk/guidance/ipg114
- Endobronchial valve insertion to reduce lung volume in emphysema. [Internet]. National Institute for Health and Care Excellence; Available online: https://www.nice.org.uk/guidance/ipg600
- Multidisciplinary Team Meeting. NHS Business Definitions. In. Available online: https://www.datadictionary.nhs.uk/data_dictionary/nhs_business_definitions/m/multidisciplinary_team_meeting_de.asp?shownav=1
- Haward RA. The Calman-Hine report: a personal retrospective on the UK’s first comprehensive policy on cancer services. Lancet Oncol 2006;7:336-46. [Crossref] [PubMed]
- McNulty W, Jordan S, Hopkinson NS. Attitudes and access to lung volume reduction surgery for COPD: a survey by the British Thoracic Society. BMJ Open Respir Res 2014;1. [Crossref] [PubMed]
- Zoumot Z, Jordan S, Hopkinson NS. Emphysema: time to say farewell to therapeutic nihilism. Thorax 2014;69:973-5. [Crossref] [PubMed]
- Buttery S, Lewis A, Oey I, et al. Patient experience of lung volume reduction procedures for emphysema: a qualitative service improvement project. ERJ Open Res 2017.3. [PubMed]
- Mahadeva R, Zoumot Z. Living with COPD: the struggle for breath and for lung volume reduction therapies. ERJ Open Res 2018.4. [PubMed]
- Chew J, Herre J, Perrott S, et al. P199 A multidisciplinary copd hyperinflation service: report of decision outcomes. Thorax 2016;71:A192-3. [Crossref]
- Marin JM, Carrizo SJ, Gascon M, et al. Inspiratory capacity, dynamic hyperinflation, breathlessness, and exercise performance during the 6-minute-walk test in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:1395-9. [Crossref] [PubMed]
- Karia S, Mahadeva R, Balan A, et al. The evolving role of MDCT in the assessment of patients with chronic obstructive pulmonary disease. Clin Radiol 2015;70:752-9. [Crossref] [PubMed]