Multiple primary lung cancers
The January issue of The Journal of Thoracic and Cardiovascular Surgery recently published an article which demonstrates the high discrepancy of genetic features in patients with multiple pulmonary tumors. The article titled “Favorable prognosis and high discrepancy of genetic features in surgical patients with multiple primary lung cancers” by Chen et al. (1) is another valuable retrospective study evaluating patients treated for multiple primary cancers. In addition to survivorship data, the group reviewed driver mutations within the multiple tumors showing highly discordant mutations arguing that the lesions represent separate primary tumors of the lung as opposed to metastatic tumors. This article brings to the forefront issues frequently seen in patients with multiple pulmonary lung cancers.
Multiple primary lung cancers (MPLC) include two subsets of patients frequently referred to as synchronous multiple primary lung cancers (SMPLC) and metachronous multiple primary lung cancers (MMPLC). Stedman’s Medical Dictionary (2) defines synchronous as “occurring simultaneously” and metachronous as “not synchronous; multiple separate occurrences such as multiple primary cancers developing at intervals”. The terminology allows for confusion and variability in outcome data. Chen’s recent paper (1) was well written, insightful, and well-conceived but still only refers to the cases as MPLC without reporting SMPLC or MMPLC. Presumably both were included.
As a result, studies have reported a large range of outcomes in patients with multiple lung malignancies that further confuses clinicians. Published 5-year survival for SMPLC ranges between 10% (3) and 76% (1) and often cited as 30% (4). Obviously, there is variability in inclusion criteria which creates these dramatic differences in outcomes. Chen et al. (1) showed in their recent study that 5-year overall survival was 76%. However, in one group, patients had 2 or more non-solid GGO’s which were either carcinoma in situ or minimally invasive adenocarcinomas. This group’s 5-year recurrence free survival was 100%. Obviously, these patients are going to do much better and arguably should have not been included. In the same study patients with 2 solid primary lung cancers resected had recurrence-free survival of 60% at 5 years.
In 2011 we published a multi institutional study (5) looking at SMPLC following very strict criteria and excluded Bronchoalveolar Tumors, GGOs, and ipsilateral tumors of same histology. Our results showed cancer specific 3- and 5-year survivals were 73% and 69%. Survival between bilateral and ipsilateral tumors was not different in this series. The intention of this study was to exclude tumors that might falsely show improved outcomes and to exclude metastatic lesions that may falsely show worse outcomes. It is my belief that this multicenter paper most accurately depicts cancer survivorship of SMPLC.
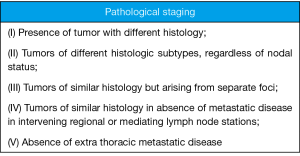
In 1975 Martini and Melamed (6) suggested criteria by which multiple malignancies within lung parenchyma should be considered multiple primary tumors as opposed to metastasis. These principles were developed from a practical perspective and remain useful today. Tumors are considered unrelated if they are either different histologically if same histology they must not have metastatic disease to a shared lymph node basin and no distant metastatic disease. The article included only 18 patients with SMPLC the remainder were MMPLC. These guidelines have stood the test of time and remain the primary guide in the setting of clinical staging and preoperative planning and I see little need for change. Pathological staging may however be a different issue. Pathologic staging is established after resection of all tumors and can dramatically influence postoperative adjuvant treatment, prognosis, and additional studies. An alternative pathologic staging system has been described by David Finley (7). I have modified those criteria further and listed them in Figure 1. This criteria should be applied following resection. I endorse these as the new standard for defining SMPLC and MMPLC pathologically.
Frequently patients seen with two or more suspicious parenchymal lesions are considered metastatic and not as SMPLC. Unfortunately, this results in death due to the inability of chemotherapy to cure primary lung cancer alone. But data is now available that suggests most of these patients have multiple primaries not metastatic disease. It appears that our eagerness to label patients with 2 or more lesions as T4 (ipsilateral different lobes) or M1a (contralateral lung) is probably wrong and misplaced. Although Chen’s paper (1) did not delve into this issue its seems obvious that the high discordance between tumor mutations demonstrates that multiple tumors in the lung parenchyma are usually unrelated not usually metastatic. In Chen’s paper utilizing eight driver mutations he found most tumors represented independent primaries. In their study driver mutations were discordant in 35 of 39 (89.7%) of patients and 94.6% discordant in patients harboring at least 1 of the detected driver mutations. These percentages are similar to Finley’s paper (7) which used histologic subtyping. Their study showed of 175 cases only 7 (4%) were considered the same while 168 (96%) were thought to be unrelated. Of 141 cases of tumors in the same histology only 7 (5%) were considered the same.
Patients with MPLC face many challenges one of which is simply being staged appropriately. Once staged appropriately they should be offered surgery because of high 5-year cancer free survival (5). If we apply Finley’s pathologic definition of MPLC (7) and look at patients as potentially operable we can offer many more patients therapy with curative intent.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Chen K, Chen W, Cai J, et al. Favorable prognosis and high discrepancy of genetic features in surgical patients with multiple primary lung cancers. J Thorac Cardiovasc Surg 2018;155:371-9.e1. [Crossref] [PubMed]
- Stedman. Stedman's Medical Dictionary for the Health Professions and Nursing. 5th edition. Baltimore: Lippinocott, Williams & Williams; 2005.
- Aziz TM, Saad RA, Glasser J, et al. The management of second primary lung cancers. A single centre experience in 15 years. Eur J Cardiothorac Surg 2002;21:527-33. [Crossref] [PubMed]
- Detterbeck FC, Rivera MP, Socinski MA, et al. Diagnosis and treatment of lung cancer: an evidence-based guide for the practicing clinician. Philadelphia: W.B. Saunders Co.; 2001.
- Fabian T, Bryant AS, Mouhlas AL, et al. Survival after resection of synchronous non-small cell lung cancer. J Thorac Cardiovasc Surg 2011;142:547-53. [Crossref] [PubMed]
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [PubMed]
- Finley DJ, Yoshizawa A, Travis W, et al. Predictors of outcomes after surgical treatment of synchronous primary lung cancers. J Thorac Oncol 2010;5:197-205. [Crossref] [PubMed]