Surgical closure of atrial septal defects
Introduction
Since the early reports of surgical atrial septal defect (ASD) closure in 1948 (without direct visualization) and in 1952 (with hypothermia and inflow occlusion), over 50 years of surgical experience has resulted in an operation with minimal mortality or morbidity (1,2). Today ASDs are closed using cardiopulmonary bypass (CPB) with direct vision of the lesion. The classic approach is by median sternotomy, however, other approaches are used as well in an effort to reduce morbidity (3). Modern minimal access techniques such as video-assisted thoracoscopy and robotic surgery are now safe and reproducible with improved cosmetic results and shortened recovery (4,5).
For isolated secundum defects, surgery can be performed with near-zero mortality. Major morbidity can include arrhythmia, pneumothorax, bleeding, pericardial effusions and pleural effusions but are usually transient. In adults and elderly patients arrhythmia and prolonged intensive care unit stay are more common (6). The long-term results of surgical repair of secundum defects are excellent, especially when patients are operated on under 25 years of age with an actuarial survival curve identical to the general population (7).
Classification of ASDs
Ostium secundum defect
Defects of septum primum tissue within the fossa ovalis are termed Secundum ASDs. The septum secundum typically remains well developed. Most secundum defects are separate from the atrioventricular valves, vena cava, pulmonary veins, and the coronary sinus. It is the most common ASD if patent foramen ovale is not considered and can range in size from only millimeters to 2–3 cm. Large defects are due to complete absence of the septum primum.
Ostium primum defect
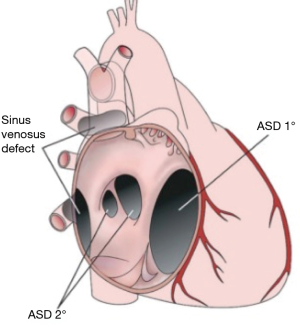
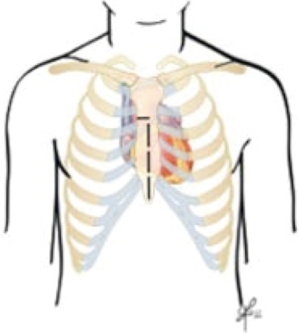
Ostium primum ASDs are a variant of atrioventricular septal defects and typically occur after failure of superior and inferior endocardial cushion fusion. The interatrial defect is situated between the anterior-inferior margin of the fossa ovalis and the atrioventricular valves. There are two distinct atrioventricular valve annuli, however, the left atrioventricular valve is typically malformed with a large cleft in the anterior mitral leaflet, dividing it into two. The position and course of the conduction axis is also abnormal and is similar to that seen in atrioventricular septal defects (Figure 1).
Sinus venosus defect
This defect results from abnormal resorption of the sinus venosus tissue that forms the junction between the vena cava and the heart. About 10% of ASDs are sinus venosus defects (8). The most common form is the superior vena cava (SVC) type (90%), where there is a high ASD within the sinus venarum and typically there is a deficiency of tissue between the right upper pulmonary vein and the SVC allowing a partial anomalous pulmonary venous connection (9,10). In the inferior form of this defect the communication between the atria is situated at the inferior vena cava—right atrial junction, and it may involve the right middle or lower pulmonary veins (Figure 1) (9,10).
Coronary sinus defect
Coronary sinus defect or unroofed coronary sinus syndrome is when all or part of the common wall between the left atrium and coronary sinus is absent. There is often an associated left SVC which connects to the coronary sinus and allows for a large right to left shunt. Whilst uncommon, this defect is often seen in situations of heterotaxy syndrome.
Common atrium
Common atrium is a rare condition that occurs when both the septum primum, septum secundum and the superior and inferior endocardial cushions fail to develop. It is usually seen in patients with heterotaxy syndrome (Figure 1).
Treatment
ASD closure is indicated in the presence of any hemodynamically significant shunt causing enlargement of right heart structures, irrespective of the presence of symptoms (11,12). A hemodynamically significant shunt is classically defined as any shunt that causes right-sided volume overload and pulmonary over-circulation. This typically occurs when the Qp:Qs ratio is greater than 1.5 to 1. Other indications for ASD closure include the rare cases of documented orthodeoxia-platypnea—regardless of shunt size, and confirmed paradoxical embolism (12). Small defects without evidence of right heart volume overload may be followed without surgery, however, increased shunt may occur later in life and closure become necessary.
In some situations, it may be unsafe to close an ASD. European and American guidelines suggest that ASDs can be closed if the pulmonary vascular resistance is less than two-thirds of the systemic vascular resistance and the Qp:Qs is greater than 1.5 (11,12). An interatrial right-to-left shunt such as in Eisenmenger syndrome is a contraindication for ASD closure and patients with a calculated pulmonary vascular resistance greater than 8 Woods units should undergo a pulmonary vasodilator challenge or a course of anti-pulmonary hypertensive medication to determine reversibility of pulmonary vascular resistance and thus operability (13). High risk patients with pulmonary hypertension may even require temporary balloon occlusion of the defect with hemodynamic assessment. In severe obstructive or restrictive right and left heart lesions closure of an ASD may also be contraindicated as it serves as a decompressing route for blood flow (12,14).
Timing of defect closure
Once a hemodynamically significant ASD is diagnosed then it should be closed electively. There is no lower age limit for defect closure, however, it is customary to refer for surgery prior to school age. We offer elective surgery as early as two years old and preferably before 5 years of age. Even in adults and the elderly, ASD closure is safe and improves symptoms and longevity (6,13,15,16).
Treatment strategies
Secundum defects can be closed surgically or percutaneously using a catheter delivered device. Limitations to transcatheter closure generally relate to the size of defects (too large) or the size of infants (too small). Relative contraindications to device closure of secundum defects include very large size (diameter greater than 36–40 mm), inadequate margins for device anchorage, potential device interference with atrioventricular valve function, and potential obstruction of systemic or pulmonary venous drainage (17,18). Sinus venosus, ostium primum, and coronary sinus septal defects almost always require surgical repair.
Surgical techniques
Secundum ASD
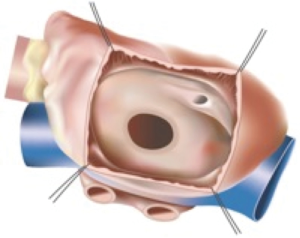
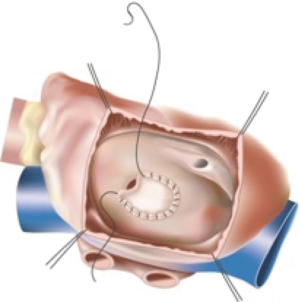
For an uncomplicated secundum defect the intracardiac repair is relatively simple. The right atrium can be opened longitudinally or transversely and small ASDs or PFOs can be closed by direct suture. Larger defects usually require a patch repair. The traditional approach through a median sternotomy and central cannulation has become exceedingly safe, simple and reproducible. Therefore, advancements in this operation have related to surgical access, in an attempt to reduce trauma and hasten recovery (Figures 2 and 3).
Sinus venosus ASD
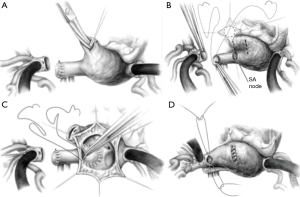
Because this defect is typically related to the septum adjacent to the vena cava, the situation of partial anomalous pulmonary venous drainage is often present. Most commonly the right upper and/or middle pulmonary veins drain connect to the atrium or SVC anomalously. If the anomalous pulmonary venous drainage is to the atrium and adjacent to the ASD then the repair is simple and the same as for secundum ASDs. More often, anomalous pulmonary veins drain to the SVC and either a two-patch or a Warden technique are required. The two-patch technique uses one patch to close the ASD and one patch to close the right atriotomy at the cavoatrial junction in such a fashion as to avoid SVC to right atrial stenosis. The right atrial incision often extends onto the SVC and the sinoatrial node is at risk for consequent dysfunction (19). In the Warden technique the SVC is divided above the anomalous pulmonary veins and anastomosed to the right atrial appendage. The residual SVC now receiving only pulmonary venous blood is baffled to the ASD, and in the process separates the atria (19). This technique avoids a cavoatrial incision and the risk of sinus node dysfunction, however, late SVC obstruction at the right atrial anastomosis has been described and one must be sure to adequately resect the pectinate muscles inside the right atrial appendage to avoid this complication (20). Modifications of the Warden technique include; using the right atrium as a superior based flap to the SVC with an anterior pericardial patch, or direct posterior SVC to right atrial anastomosis with an anterior pericardial patch, these techniques are aimed at avoiding tension and stenosis, especially in patients with high pulmonary vein to SVC entry (Figure 4A,B,C,D).
Surgical approach
Median sternotomy
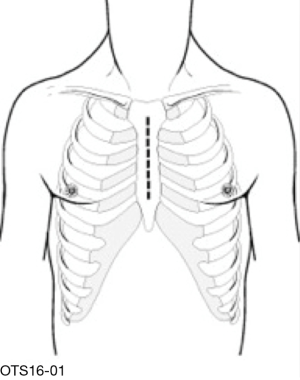
Median sternotomy is typically thought to be the gold-standard approach and provides the ability to perform any additional cardiac procedure. Skin incision length can be shortened at both the cranial and caudal ends to allow better cosmesis, but the sternum is usually divided in its entirety and thus the requisite recovery unavoidable. Mechanical ventilation and ICU stay times are short and intraoperative time, CPB time is typically shorter using this exposure. Cannulation is via the ascending aorta and both cava directly (Figure 5).
Partial or mini-sternotomy
In this technique, a small midline incision is used and the sternum is only partially divided. This can be in the form of a “T” shape, inverted “J” shape, or in infants and toddlers with malleable cartilaginous sternums, the sternum may only need to be divided to the level of the manubriosternal junction.
Advantages include less postoperative chest tube drainage, shorter stay in the hospital, and better cosmetic effect, especially in young women (21). This was not proven in pediatric series (Figure 6).
Right anterolateral thoracotomy
The right atrium was traditionally approached from a standard right posterolateral thoracotomy. The incision is in the line of the ribs and muscle is divided, however, this was a large and often painful incision, and is rarely used nowadays. Modern modifications include the anterolateral and axillary vertical incision muscle sparing approaches. Once inside the right pleural space the right thymic lobe can be excised or mobilized anteriorly. The pericardium is then opened at distance from the phrenic nerve and traction sutures used on both sides. Each subsequent pericardial stay suture gradually improves visualization. The superior two sutures can be tied to the periosteum of the rib which this pulls the ascending aorta towards the surgeon and facilitates cannulation.
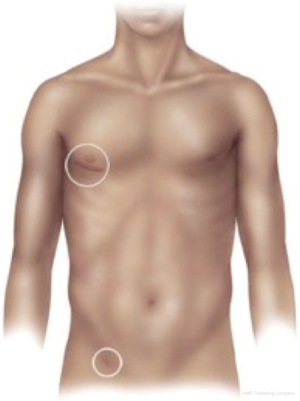
The right anterolateral thoracotomy has become a popular approach because of its extensive use in adult minimally invasive mitral repair. This approach is best used in females once the breast bud has developed as the incision can be hidden in the breast crease. Good cosmetic and functional results have increased its popularity (22-24).
The patient is typically positioned on the operating table with their right side elevated by a sand bag under the shoulder. The right groin vessels should always be left free in case peripheral cannulation is required for bypass. The incision is short: medially it stops about 2–3 cm from the lateral edge of the sternum, and laterally it continues to the anterior axillary line. When it runs in the breast crease in women it is virtually invisible postoperatively as the breast tissue overlies the scar. The fourth intercostal space is opened. If the aorta is deep and not easily accessible the femoral artery is cannulated. The fifth intercostal space can be preferentially used if peripheral arterial and venous cannulation is pre-planned. A smaller incision can be used if shafted instruments are used and peripheral cannulation and ventricular fibrillation applied, as this minimizes the amount of working space taken up by cannula, cross-clamp, and cardioplegia lines running through the incision. In prepubescent girls significant asymmetrical breast development occurs in only 5% of patients with 98% of patients satisfied with the cosmetic result (22). However, other groups have described up to 20% rates of asymmetrical breast development if operated on before puberty although with low risk of scoliosis (25) (Figure 7).
Right vertical axillary thoracotomy
For a vertical axillary incision, the patient is positioned in the left lateral decubitus position, but the hips are placed at 45 degrees to enable access to femoral vessels. Skin flaps need to be created to allow incision mobility, then a plane is developed to allow retraction of the latissimus dorsi muscle, long thoracic nerve and lateral thoracic vessels posteriorly. The third and fourth rib are exposed by going between fibers of the serratus anterior muscle. Lung isolation also facilitates chest entry and lung retraction. The third intercostal space gives better access to the aorta and SVC in infants and small children, however, the fourth or fifth intercostal space gives better access to the IVC and is usually the better option for straight forward ostium secundum defects. In larger children and adolescents, the aorta is more remote and aortic cannulation, insertion of a cardioplegia needle and cross-clamp positioning can be challenging. Therefore, peripheral arterial and venous cannulation is performed using the right groin vessels, and ventricular fibrillation used to facilitate intracardiac access
The advantages of the vertical axillary incision include; a shorter recovery and time to return of functional capacity of the right arm and shoulder, its location away from breast tissue with low risk of asymmetric breast development, no risk of scoliosis, and a scar completely hidden by the resting arm (26). The main risk of this incision is damage to the intercostobrachial nerve and subsequent loss of sensation of the ipsilateral thoracic wall (26) (Figure 8).
Video-assisted thoracoscopic surgery (VATS)
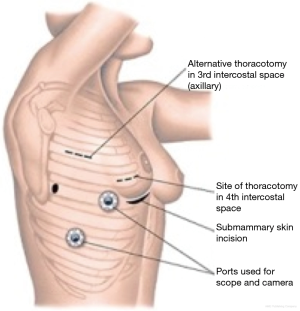
VATS requires general anesthesia with a double lumen endotracheal tube or bronchial blocker. The patient is placed at 30 degree elevation of the right side. A 2–3 cm incision is then made in the right inframammary crease and the chest is entered in the fourth intercostal space. A 5-mm thoracoscope is placed lateral to this incision. Peripheral cannulation is preferentially used with a percutaneous approach to the right jugular vein and open exposure of the femoral vessels to institute bicaval CPB. A stab incision lateral to the right internal mammary artery allows insertion of the cardioplegia line. Specially shafted long instruments are required and vision of the procedure is primarily on a video monitor rather than through the incision. The right atrium is opened and ASD repaired in the same fashion as above. Usually a direct closure is performed, however, when a patch is required bovine pericardium is used as it is easier to work with and there is no need to harvest autologous pericardium.
Contraindications to this approach include patients with other cardiac conditions which need to be repaired and prior right thoracotomy. A patient weight above 20 kg is usually required to allow appropriate intercostal space for instrumentation. Unfortunately this typically represents children above 5–6 years of age, and most western units would operate on these patients before 5 years of age (27) (Figure 9).
Robotic
The robotic approach is a similar set up to VATS. General anesthesia with either a double lumen tube or endobronchial blocker for single lung ventilation is required. The patient is placed in a 30 degree right sided position and a right internal jugular venous sheath is placed and prepped into the sterile surgical field. The sheath provides access for percutaneous jugular venous cannulation for CPB. The right arm is tucked posterior to the axillary line to allow for transthoracic aortic cross clamp placement. The head and neck must be well supported to avoid brachial plexus strain. The incisions and port placements are similar to robotic mitral valve repair incisions (28). The robotic surgeon and another bedside cardiac surgeon work together to ensure a safe and efficient procedure. The right femoral vessels are exposed, the right lung is deflated and an incision is made in the right inframammary crease with entry into the pleural cavity through the fourth intercostal space. A peripheral catheter can be placed medial to the incision for the instillation of CO2. The pericardium is opened in the usual fashion anterior to the phrenic nerve and pericardial sutures are placed on the inferior edge and pulled through a needle hole laterally at the axillary line.
A long cardioplegia cannula is placed through the inframammary incision and the aorta is cross-clamped through a separate stab incision. The intracardiac repair is the same as above however all instruments apart from the knot tying device are controlled via robotic arms, with the primary surgeon unscrubbed sitting at a console controlling the instruments, all vision is provided by video thoracoscopy (Figure 10).
The integrity of the repair and adequacy of de-airing is confirmed by transesophageal echocardiography and a bubble study is typically performed to rule out any residual shunt. Decannulation and reversal of heparin are performed in the usual manner. The pericardium is loosely re-approximated and the chest and femoral wounds are closed in layers.
The advent of VATS, with or without robotic assistance, avoids the disadvantages of conventional full sternotomy and is reported to be safe and effective, with no mortality and very low morbidity (29). It requires experience in both robotic and port-access techniques and as well as perfusion strategy. Improvement in postoperative recovery, expeditious return to work as well as improved cosmesis are evidence that a VATS or robotic approach can be cost effective despite increased operating room time and costs (30).
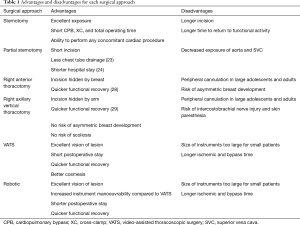
The evolution of robotic cardiac surgery in children has been slower than in adults, particularly for intracardiac procedures. Firstly, the smaller thoracic cavity and narrower intercostal spaces in children increases the risk of contact between instruments during surgery and the risk of intercostal nerve injury causing prolonged postoperative pain. Secondly, CPB in pediatric patients can be difficult even with full sternotomy exposure and peripheral cannulation is not always ideal or feasible. Thirdly, the ischemic time is prolonged using minimally invasive techniques, with subsequent risk to myocardial function. The large size of current robotic systems also limits usage of this technology in small children (31). Newer generation models are being developed which use portholes of 5–8 mm as compared with earlier versions for which the portholes were 10 mm. However, robotic systems are not routinely used by pediatric cardiac surgeons, due to their high cost, lack of pediatric sized instruments, and the small chest volumes of children that limit the working space. This is despite its definite advantages, including superior intrathoracic range of motion, improved visualization at angles, and the cosmetic advantages of small skin incisions (32) (Table 1).
Full table
Conclusions
The surgical treatment of ASDs in the modern era of cardiac surgery has many faces. The traditional sternotomy approach remains the easiest technique with the shortest ischemic and CPB times. Modern minimally invasive approaches improve cosmesis, shorten hospital stay, hasten return to full function, and can now be performed without increased risk in terms of mortality and morbidity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Murray G. Closure of defects in cardiac septa. Ann Surg 1948;128:843-53. [Crossref]
- Lewis FJ, Taufic M. Closure of atrial septal defects with the aid of hypothermia; experimental accomplishments and the report of one successful case. Surgery 1953;33:52-9. [PubMed]
- Nagendran J, Habib HF, Kiaii B, et al. Minimally invasive endoscopic repair of atrial septal defects via right minithoracotomy. Multimed Man Cardiothorac Surg 2016;2016.
- Suematsu Y, Kiaii B, Bainbridge DT, et al. Robotic-assisted closure of atrial septal defect under real-time three-dimensional echo guide: in vitro study. Eur J Cardiothorac Surg 2007;32:573-6. [Crossref] [PubMed]
- Burkhart HM, Suri RM. Minimally invasive video assisted surgical closure of secundum atrial septal defect. Ann Cardiothorac Surg 2017;6:60-3. [Crossref] [PubMed]
- Nyboe C, Fenger-Gron M, Nielsen-Kudsk JE, et al. Closure of secundum atrial septal defects in the adult and elderly patients. Eur J Cardiothorac Surg 2013;43:752-7. [Crossref] [PubMed]
- Murphy JG, Gersh BJ, McGoon MD, et al. Long-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 years. N Engl J Med 1990;323:1645-50. [Crossref] [PubMed]
- Geva T, Martins JD, Wald RM. Atrial septal defects. Lancet 2014;383:1921-32. [Crossref] [PubMed]
- Van Praagh S, Carrera ME, Sanders SP, et al. Sinus venosus defects: unroofing of the right pulmonary veins--anatomic and echocardiographic findings and surgical treatment. Am Heart J 1994;128:365-79. [Crossref] [PubMed]
- Banka P, Bacha E, Powell AJ, et al. Outcomes of inferior sinus venosus defect repair. J Thorac Cardiovasc Surg 2011;142:517-22. [Crossref] [PubMed]
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e143-263. [Crossref] [PubMed]
- Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57. [Crossref] [PubMed]
- Kim YH, Yu JJ, Yun TJ, et al. Repair of atrial septal defect with Eisenmenger syndrome after long-term sildenafil therapy. Ann Thorac Surg 2010;89:1629-30. [Crossref] [PubMed]
- Hanninen M, Kmet A, Taylor DA, et al. Atrial septal defect closure in the elderly is associated with excellent quality of life, functional improvement, and ventricular remodelling. Can J Cardiol 2011;27:698-704. [Crossref] [PubMed]
- Humenberger M, Rosenhek R, Gabriel H, et al. Benefit of atrial septal defect closure in adults: impact of age. Eur Heart J 2011;32:553-60. [Crossref] [PubMed]
- Nakagawa K, Akagi T, Taniguchi M, et al. Transcatheter closure of atrial septal defect in a geriatric population. Catheter Cardiovasc Interv 2012;80:84-90. [Crossref] [PubMed]
- Meier B. Percutaneous atrial septal defect closure: pushing the envelope but pushing it gently. Catheter Cardiovasc Interv 2005;66:397-9. [Crossref] [PubMed]
- Marie Valente A, Rhodes JF. Current indications and contraindications for transcatheter atrial septal defect and patent foramen ovale device closure. Am Heart J 2007;153:81-4. [Crossref] [PubMed]
- Stewart RD, Bailliard F, Kelle AM, et al. Evolving surgical strategy for sinus venosus atrial septal defect: effect on sinus node function and late venous obstruction. Ann Thorac Surg 2007;84:1651-5; discussion 1655. [Crossref] [PubMed]
- Warden HE, Gustafson RA, Tarnay TJ, et al. An alternative method for repair of partial anomalous pulmonary venous connection to the superior vena cava. Ann Thorac Surg 1984;38:601-5. [Crossref] [PubMed]
- Luo W, Chang C, Chen S. Ministernotomy versus full sternotomy in congenital heart defects: a prospective randomized study. Ann Thorac Surg 2001;71:473-5. [Crossref] [PubMed]
- Vida VL, Padalino MA, Boccuzzo G, et al. Minimally invasive operation for congenital heart disease: a sex-differentiated approach. J Thorac Cardiovasc Surg 2009;138:933-6. [Crossref] [PubMed]
- Vida VL, Tessari C, Fabozzo A, et al. The evolution of the right anterolateral thoracotomy technique for correction of atrial septal defects: cosmetic and functional results in prepubescent patients. Ann Thorac Surg 2013;95:242-7. [Crossref] [PubMed]
- Abdel-Rahman U, Wimmer-Greinecker G, Matheis G, et al. Correction of simple congenital heart defects in infants and children through a minithoracotomy. Ann Thorac Surg 2001;72:1645-9. [Crossref] [PubMed]
- Bleiziffer S, Schreiber C, Burgkart R, et al. The influence of right anterolateral thoracotomy in prepubescent female patients on late breast development and on the incidence of scoliosis. J Thorac Cardiovasc Surg 2004;127:1474-80. [Crossref] [PubMed]
- Nguyen K, Chin C, Lee DS, et al. The axillary incision: a cosmetic approach in congenital cardiac surgery. J Thorac Cardiovasc Surg 2007;134:1358-60. [Crossref] [PubMed]
- Bacha E, Kalfa D. Minimally invasive paediatric cardiac surgery. Nat Rev Cardiol 2014;11:24-34. [Crossref] [PubMed]
- Suri RM, Burkhart HM. Optimizing outcomes of robotic mitral valve repair for all prolapse anatomy: the Suri-Burkhart technique. Ann Cardiothorac Surg 2013;2:841-5. [PubMed]
- Bonaros N, Schachner T, Oehlinger A, et al. Robotically assisted totally endoscopic atrial septal defect repair: insights from operative times, learning curves, and clinical outcome. Ann Thorac Surg 2006;82:687-93. [Crossref] [PubMed]
- Suri RM, Thompson JE, Burkhart HM, et al. Improving affordability through innovation in the surgical treatment of mitral valve disease. Mayo Clin Proc 2013;88:1075-84. [Crossref] [PubMed]
- Burke RP, Hannan RL. Reducing the trauma of congenital heart surgery. Surg Clin North Am 2000;80:1593-605. [Crossref] [PubMed]
- Suematsu Y, del Nido PJ. Robotic pediatric cardiac surgery: present and future perspectives. Am J Surg 2004;188:98S-103S. [Crossref] [PubMed]