Endobronchial ultrasound-guided versus conventional transbronchial needle aspiration: time to re-evaluate the relationship?
Conventional transbronchial needle aspiration (cTBNA) has a long history over three decades of utility for mediastinal sampling in lung cancer (1) especially in more bulky disease and can achieve high yields (nearly 80% or above) even in relatively new services (2). It has also been utilised in the diagnosis of benign mediastinal disease (3). The attractiveness of cTBNA is that it can be performed at the same sitting as conventional bronchoscopy by a respiratory physician/interventional pulmonologist under conscious sedation in an endoscopy suite without needing a thoracic surgeon, operating theatre, theatre team and associated expenses (4). A common application of cTBNA is to select out those patients with multi-station or bulky N2 disease who are not suitable for radical therapy but would be suitable for oncological therapies and thereby avoiding the invasiveness and cost of mediastinoscopy for diagnosis alone. More recently, in the last decade or so (5,6), endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has come to the fore which has prompted a re-evaluation of all mediastinal sampling and staging techniques (4,7). There has also been further interest in the utility of both cTBNA and EBUS-TBNA beyond lung cancer in diagnosis of treatable benign mediastinal disease such as tuberculosis (3,6).
What is of interest however is how the relative roles of cTBNA and EBUS-TBNA have evolved and what they should be when the expertise and equipment is available in the same centre. Given the technical advantages of EBUS-TBNA [real-time sampling, imaging of surrounding vessels and nodal size and nature (8)], intuitively EBUS-TBNA should be superior for smaller nodes or those in more remote locations or juxtaposed to vessels. There are now several studies in the literature (albeit mainly retrospective or observational) that have compared the two techniques in both lung cancer and granulomatous disease (see Tables 1 and 2). The main findings consistently have been either superiority of diagnostic yield, sample adequacy or safety for EBUS-TBNA over cTBNA with variations depending on node size (smaller nodes favouring EBUS-TBNA) or location (station 7 often equivalent for both).
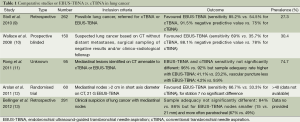
Full table

Full table
It is in this context that the paper in this issue of Journal of Thoracic Disease by Jiang et al. (16) attempts to compare the relative utilities of the two techniques in lung cancer. They report that cTBNA was non-inferior to EBUS-TBNA in lung cancer patients with mediastinal nodes. Unfortunately, the paper lacks other important data that would allow greater interpretation of the results and putting them in context. Firstly, the yields reported for EBUS-TBNA in the paper are low at ~78% not in keeping with published results of 88-93% (6,7) even in new services (17,18). This may partially relate to the particular cohort with lower cancer prevalence due to ethnicity and also reflect the utility of these techniques in benign disease as in the real world but further conclusions cannot be drawn as there are no data on node size, node location or staging especially given the limitations of positron emission tomography (PET) in radiological staging in sensitivity for mediastinal metastases. Potentially larger and more central nodes (not stations 2R, 2L) would favour cTBNA results as cTBNA would be expected to match EBUS-TBNA here. There are no data provided on surgical confirmation of results or clinical follow-up times which allow verification and clarification of “true negative” and “false negative” TBNA samples and identification of an alternative (benign) pathology. Learning curves for EBUS-TBNA may also be relevant here (discussed later on).
Secondly, they report 34 patients did not tolerate EBUS-TBNA (nearly 12% of the original cohort) which is far higher than would normally be expected in what is usually a well-tolerated procedure under conscious sedation (19,20). No information is provided on dosage of sedation, presence of an anaesthetist or procedure time but the fact that EBUS-TBNA was consistently performed after cTBNA may have been a factor. This also introduces bias of bronchoscopic landmarks for cTBNA puncture sites (for initial localisation prior to optimising position with the ultrasound) and also a small but theoretical risk of cross contamination of the EBUS-TBNA samples as the order was not randomised [a 7% rate of false positivity has been described with cTBNA (21)]. The non-inferior yield and poor patient tolerance of EBUS-TBNA compared to cTBNA may also reflect the ten times greater experience with cTBNA than for EBUS-TBNA in the study centre and it would be interesting to see a repeat study after longer experience with EBUS-TBNA to see if the results change. Existing studies have demonstrated the learning curve for EBUS-TBNA can be longer than thought and also individualised even amongst experienced conventional bronchoscopists (22,23). This is not surprising bearing in mind the endoscopic image is off-set with many systems and the scope is heavier and more rigid to manipulate (8).
Other limitations on the dataset include the fact that only one pathologist who not blinded to the study and reported all the results (it is common place now for double reporting of all lung pathology samples) so they were not analysed in blind fashion or independently which limited the internal validity. Only one to three samples were taken per station which may have lowered the sensitivity overall as standard practice suggest at least one visible core or three samples minimum should be taken per station (24). Moreover, the average number of samples taken was not provided and should be balanced to avoid bias as the change in sensitivity varies from 69% to 95% between one and three samples (24). There is no data provided on safety mid-procedure for both cTBNA and EBUS-TBNA which is of relevance as EBUS-TBNA would be expected to avoid vascular puncture due to real-time sampling.
Other factors that would have enhanced the study include data on ability to perform growth factor mutation analysis for epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) as individualised treatment based on mutation status is now the aim of oncological therapy. It is known that EBUS-TBNA for example reports a high degree of success with providing samples for EGFR mutation testing (25,26). Additional information on needle gauge for both cTBNA and EBUS-TBNA would have been of interest as, depending on pathology set-up, there is more recent evidence that 21G EBUS-TBNA samples provide better subcharacterisation than 22G samples in a histopathological setup (18). Information on what benign diagnoses were made in the cancer negative samples would also been of interest to evaluate the performance of cTBNA and EBUS-TBNA in benign disease. In sarcoidosis for example, there is good randomised trial evidence to favour EBUS-TBNA over sarcoidosis (14). A cost analysis would also have been of interest given that the setup costs for EBUS-TBNA are far higher than for cTBNA but the importance of accurate clinical coding for these activities is even more important given the potential implications for loss of activity-based revenue in some healthcare systems which can be avoided by greater physician interaction with coders (2,27).
So what conclusions can be drawn from the Jiang et al. (14) study? In reality, this study has raised more questions than answers. It would be interesting to see a follow-up study to see if there is a learning effect with EBUS-TBNA as per cTBNA. Future randomised studies are needed but including data on follow-up, surgical confirmation, node size and location to definitively answer the question but one suspects the techniques should be complementary as suggested by national bodies (28). In the meantime, we know that cTBNA can perform well in selected patients in centres with relevant expertise but we do not have the evidence to conclude that cTBNA is non-inferior to EBUS-TBNA in all situations. However, we can infer cTBNA is non-inferior in the scenario of bulky or central mediastinal disease which is the probable context of the Jiang et al. study (16). In this respect, it is worth continuing to use cTBNA in centres with relevant expertise where the cost of setting up an EBUS-TBNA would be prohibitively high but EBUS-TBNA should be used for mediastinal staging of smaller nodes and at more distal nodal stations if available.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Wang KP, Brower R, Haponik EF, et al. Flexible transbronchial needle aspiration for staging of bronchogenic carcinoma. Chest 1983;84:571-6. [PubMed]
- Medford AR, Agrawal S, Free CM, et al. A prospective study of conventional transbronchial needle aspiration: performance and cost utility. Respiration 2010;79:482-9. [PubMed]
- Wang KP, Fuenning C, Johns CJ, et al. Flexible transbronchial needle aspiration for the diagnosis of sarcoidosis. Ann Otol Rhinol Laryngol 1989;98:298-300. [PubMed]
- Medford AR, Bennett JA, Free CM, et al. Mediastinal staging procedures in lung cancer: EBUS, TBNA and mediastinoscopy. Curr Opin Pulm Med 2009;15:334-42. [PubMed]
- Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest 2004;125:322-5. [PubMed]
- Medford AR, Bennett JA, Free CM, et al. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA): applications in chest disease. Respirology 2010;15:71-9. [PubMed]
- Low A, Medford AR. Endobronchial ultrasound-guided transbronchial needle aspiration. Rev Recent Clin Trials 2013;8:61-71. [PubMed]
- Medford AR. Convex probe endobronchial ultrasound: pitfalls, training and service issues. Br J Hosp Med (Lond) 2011;72:312-7. [PubMed]
- Stoll LM, Yung RC, Clark DP, et al. Cytology of endobronchial ultrasound-guided transbronchial needle aspiration versus conventional transbronchial needle aspiration. Cancer Cytopathol 2010;118:278-86. [PubMed]
- Wallace MB, Pascual JM, Raimondo M, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008;299:540-6. [PubMed]
- Rong F, Xiao SH, Liu J, et al. A comparative research on the conventional transbronchial needle aspiration and endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of mediastinum lesions. Zhonghua Jie He He Hu Xi Za Zhi 2011;34:120-2. [PubMed]
- Arslan Z, Ilgazli A, Bakir M, et al. Conventional vs. endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of mediastinal lymphadenopathies. Tuberk Toraks 2011;59:153-7. [PubMed]
- Bellinger CR, Chatterjee AB, Chin R Jr, et al. Conventional and endobronchial ultrasound-guided transbronchial needle aspiration: complementary procedures. South Med J 2012;105:625-9. [PubMed]
- Tremblay A, Stather DR, Maceachern P, et al. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest 2009;136:340-6. [PubMed]
- Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial Ultrasound Guided TBNA vs. Conventional TBNA in the diagnosis of sarcoidosis. Chest 2014. [Epub ahead of print]. [PubMed]
- Jiang J, Browning R, Lechtzin N, et al. TBNA with and without EBUS: a comparative efficacy study for the diagnosis and staging of lung cancer. J Thoracic Dis 2014. [Epub ahead of print].
- Medford AR, Agrawal S, Free CM, et al. A performance and theoretical cost analysis of endobronchial ultrasound-guided transbronchial needle aspiration in a UK tertiary respiratory centre. QJM 2009;102:859-64. [PubMed]
- Jeyabalan A, Shelley-Fraser G, Medford AR. The impact of needle gauge on characterisation of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) histology samples. Respirology 2014. [Epub ahead of print]. [PubMed]
- Steinfort DP, Irving LB. Patient satisfaction during endobronchial ultrasound-guided transbronchial needle aspiration performed under conscious sedation. Respir Care 2010;55:702-6. [PubMed]
- Jeyabalan A, Medford AR. Patient Satisfaction During Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Performed Under Mild Conscious Sedation. Respiration 2014. [Epub ahead of print].
- Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-220S.
- Kemp SV, El Batrawy SH, Harrison RN, et al. Learning curves for endobronchial ultrasound using cusum analysis. Thorax 2010;65:534-8. [PubMed]
- Medford AR. Learning curve for endobronchial ultrasound-guided transbronchial needle aspiration. Chest 2012;141:1643-author reply 1643-4. [PubMed]
- Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest 2008;134:368-74. [PubMed]
- Navani N, Brown JM, Nankivell M, et al. Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: a multicenter study of 774 patients. Am J Respir Crit Care Med 2012;185:1316-22. [PubMed]
- Jeyabalan A, Medford AR. Adequacy of 22 and 21 gauge EBUS-TBNA histology samples for genotyping of primary lung adenocarcinoma. Thorax 2013;68:A199-200.
- Pillai A, Medford AR. Greater physician involvement improves coding outcomes in endobronchial ultrasound-guided transbronchial needle aspiration procedures. Respiration 2013;85:417-21. [PubMed]
- Baldwin DR, White B, Schmidt-Hansen M, et al. Diagnosis and treatment of lung cancer: summary of updated NICE guidance. BMJ 2011;342:d2110. [PubMed]


