Risk factors of cough in non-small cell lung cancer patients after video-assisted thoracoscopic surgery
Introduction
Lung cancer is the most common cancer and is the leading cause of cancer-related death in China (1). With the increase in widespread general health examinations and the development of medical imaging techniques, more and more patients have been diagnosed with early-stage lung cancer and have undergone pulmonary resection (1). Cough is a common complication in patients with non-small cell lung cancer (NSCLC) who have received surgery, and the incidence ranges from 24.7% to 50.0% (2-4). In severe cough, patients experience disrupted sleep and difficulty talking, which increases their psychological burden and worsens their quality of life (4-7).
Postoperative cough has been hypothesized to be related to pulmonary C-fiber activation, extraction of the vagus nerve, acid regurgitation, endobronchial sutures and mediastinal lymph node resection as well as other anatomical reasons (3,8-11). However, the mechanism and characteristics of cough after pulmonary resection remain controversial.
Previous research has mainly focused on traditional thoracotomy rather than video-assisted thoracoscopic surgery (VATS) and has lacked adequate standardized methods for assessing postoperative cough. VATS is a widespread technique linked to improved postoperative respiratory function and reduced length of hospital stay (12-15).
In our study, we investigated postoperative cough in NSCLC patients via the Leicester Cough Questionnaire in Mandarin Chinese (LCQ-MC) and followed up with the patients at 1-month post-surgery. Meanwhile, we analyzed data to find potential risk factors that could be altered by medical intervention with the hope of reducing the incidence of postoperative cough.
Methods
This study was part of a validation of the LCQ-MC in patients undergoing lung resection for lung disease (Clinicaltrials.gov, number ChiCTR-DDD-17014237). The West China Hospital of Sichuan University in Sichuan, China, approved the study. All the participants provided written informed consent.
Subjects
A total of 198 patients who underwent thoracic surgery performed by a single medical team between September 2016 and October 2017 at the Thoracic Surgery Department of West China Hospital of Sichuan University were enrolled.
The inclusion criteria were (I) patients ≥18 years old; (II) patients with no cough symptoms experienced within 2 weeks prior to surgery; (III) patients who provided written informed consent; (IV) patients who underwent surgery performed via VATS; and (V) postoperative pathological findings indicative of NSCLC.
The exclusion criteria were (I) VATS was converted to thoracotomy or bleeding was >1,000 mL; (II) a pneumonectomy was performed; (III) the patient rejected the survey or dropped out; and (IV) the patient was diagnosed with lung cancer tumor node metastasis (TNM) stage pT4, pN3, and/or M1.
Surgical procedures
The surgery performed was a single-direction thoracoscopic lobectomy or sublobar resection, which included a segmentectomy and wedge resection of the lung (16,17). A systematic mediastinal lymphadenectomy was conducted in accordance with the Chinese Guidelines for the Diagnosis and Treatment of Primary Lung Cancer (2015 Version) (18). Double 16-French chest tubes were placed in the surgical holes in the 3rd or 4th, 7th intercostal spaces for postoperative drainage (19).
The duration of anesthesia refers to the time from the beginning of endotracheal intubation to the point of extubation. All the patients were extubated at the end of the operation and transferred to the ward after a brief stay in the recovery area.
Postoperative management
The patients were encouraged to ambulation early, and a chest X-ray was arranged on the first postoperative day. The chest tube withdrawal criteria were the absence of air leakage through the chest tube, pleural fluid drainage under 200 mL in 24 hours, and postoperative chest X-ray showing no pneumothorax. The chest tubes were removed on the secondary postoperative day if the above criteria were met.
Postoperative pain management was performed with an analgesic pump (5 mg loading dose followed by 1.0–1.5 mg/h) or using non-steroidal anti-inflammatory drugs (NSAIDs) (Tallinn or Fenbid) only when necessary. The analgesia pump is also stopped when the drainage tube is removed.
Assessment measurements
The LCQ-MC, which assesses the cough-related quality of life, consists of 19 items divided into three domains: the physical (8 items), psychological (7 items) and social (4 items) domains. A 7-point Likert scale was used to score individual domains. The total scores ranged from 3 to 21, with a higher score indicative of a better health status (20,21). In a recent study, the LCQ-MC was validated in the patients with NSCLC (22).
The minimal clinically important difference (MCID) is the smallest change in the quality-of-life score considered to be clinically meaningful (23). The MCID is 1.3 for the LCQ-MC total score relating to chronic cough (24).
The postoperative diagnoses were determined using the 2015 World Health Organization (WHO) Classification of Lung Tumors and the Eighth Edition of the Lung Cancer Stage Classification guidelines from the Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) (25,26). Regional lymph node classification was based on the International Association for the Study of Lung Cancer (IASLC) lymph node map (27,28).
Data collection
Our investigators assisted the in-patients in completing paper questionnaires in the ward. Postoperative follow-up was performed by telephone or out-patient review at one month after surgery. Then, we uploaded the data to a network database for management and analysis.
Statistical analysis
Patient characteristics are presented as the mean ± SD for continuous variables and as relative frequencies for categorical variables.
A univariate analysis was performed to identify the differences between the cough and no cough groups, using t-tests, Pearson’s χ2 tests and Yates’s correction for continuity, as appropriate.
Multivariate predictors were evaluated using logistic regression analysis, and the odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. For the logistic regression analysis, the conventional receiver operating characteristic (ROC) curve was used to determine the cutoff value of each variable that gave maximal sensitivity and specificity
All comparisons were two-sided, and differences with P≤0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS software version 19.0 (Statistical Package for the Social Sciences, Chicago, IL, USA) and GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA, USA).
Results
Patient characteristics and univariate analysis
Table 1 presents the characteristic of 198 patients diagnosed with NSCLC. Overall, 91 patients (46.0%) developed cough after surgery. These patients had a mean age of 58.33±9.69 years, and 53 of the patients were female (58.2%). The total scores of the LCQ-MC were 17.17±2.62, physical scores were 5.36±1.03, psychological scores were 5.57±1.14 and social scores were 6.23±0.87. Other baseline characteristics are indicated in Table 1.
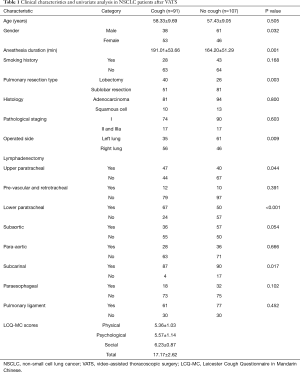
Full table
A univariate analysis was performed to determine the potential risk factors for postoperative cough. Compared to the cough group, the no cough group demonstrated significant differences in gender (P=0.032), the duration of anesthesia (P=0.001), pulmonary resection type (P=0.003), operated side (P=0.009), upper paratracheal node resection (P=0.044), lower paratracheal node resection (P<0.001), and subcarinal node resection (P=0.017), while there were no discrepancies among the other variables (Table 1).
Multivariate logistic regression analyses
After the univariate logistic regression analysis, the potential risk factors were entered into a multivariable analysis. The cutoff of anesthetic duration was 164 minutes, which was determined based on the ROC curve. In the logistic regression analysis, gender (female; OR 2.399, 95% CI: 1.260–4.565, P=0.008), duration of anesthesia (≥164 minutes; OR 2.810, 95% CI: 1.368–5.771, P=0.005), lower paratracheal node resection (OR 3.697, 95% CI: 1.439–9.499, P=0.007), and subcarinal node resection (OR 4.175, 95% CI: 1.203–14.495, P=0.024) were independent factors related to postoperative cough (Table 2). These results indicate that apart from gender, surgery-related factors were the only factors for postoperative cough.

Full table
LCQ-MC scores after surgery and follow-up
In total, 91 patients developed cough after surgery. The follow-up after 1 month demonstrated that 73 patients (80.2%) maintained their cough, 17 patients were no longer coughing (18.7%), and 1 patient (1.1%) was lost to follow-up.
The follow-up total score after 1 month (18.00±1.80) was significantly higher than the postoperative total score (16.35±2.26; P=0.004). The mean difference between the 1-month follow-up score and the postoperative score was 1.51, which was greater than the reference MCID related to chronic cough of 1.3. Meanwhile, the follow-up scores of the psychological and social domains (6.07±0.58 and 6.58±0.60, respectively) were both significantly higher than the postoperative scores (5.25±1.05 and 6.05±0.88; P<0.001 and P=0.016, respectively). However, there were no significant differences in the physical domain of the LCQ-MC between the postoperative and follow-up time points (P=0.227; Table 3). These results indicate that the postoperative cough symptoms improved after one month, especially in the psychological and social domains.

Full table
Discussion
To our knowledge, this is the first study to determine the potential risk factors of cough in NSCLC patients after VATS. Our study appears to indicate that gender (female), the duration of anesthesia, lower paratracheal node resection and subcarinal node resection were independent factors related to postoperative cough. Furthermore, we also assessed and followed up postoperative cough via the LCQ-MC, which performed satisfactorily.
Our main objective was to identify the risk factors for the development of postoperative cough following VATS in NSCLC patients. The non-surgical factor associated with an increased risk of postoperative cough was female gender, which was identified as an independent risk factors in the multivariate analysis. A recent review indicated that there was a significant gender difference in the proportion of chronic cough patients, with a preponderance of females (29). Another study demonstrated an association between the health-related quality of life (HRQOL) and women, where the HRQOL of women with chronic cough is more adversely affected than that in men, the longer a cough lasts (30). The reasons for this phenomenon may be related to hormonal influences and high visceral sensitivity in females, along with a hypersensitivity of airway afferents to the somatosensory cortex (29,31-34).
Coughing is a physiological response to airway stimulation and is associated with increases in arterial blood pressure, venous pressure and heart rate (35,36). Therefore, coughing during the induction and recovery of general anesthesia is a common phenomenon because of the tracheal stimulation during intubation and extubation (36-38). Opioids, such as fentanyl, sufentanil, and remifentanil, are widely used in the induction and maintenance of general anesthesia (39). Opioid-induced cough has been reported in many studies (40-42). In addition, the noxious effects of an anesthetic gas or of uncleared secretions are all thought to increase the incidence of coughing (43,44). Our results demonstrated that the duration of anesthesia is another important independent risk factor for postoperative cough in NSCLC patients, which is an observation consistent with previous findings.
There is a relationship between the surgical procedures and a postoperative cough. In our study, resection of the lower paratracheal nodes and resection of the subcarinal nodes were considered the most important independent risk factors indicating the development of a postoperative cough. It is possible that cough receptors are mainly located in the larynx, trachea, carina and the larger lung bronchi (45,46). Consistent with the anatomical location of cough receptors, we did find that there were significant differences in upper paratracheal node resection, lower paratracheal node resection, and subcarinal node resection between the cough group and the no cough group in the univariate analysis. Our data agree with previous studies; for example, Sawabata et al. speculated that mediastinal lymph node resection may contribute to a postoperative cough (3,10).
We administered the LCQ-MC to describe the longitudinal changes in cough symptoms. It is a useful tool that is used in clinical trials and longitudinal studies of chronic cough (11,20,21). However, there were no reports about assessing cough with the LCQ-MC in NSCLC patients after VATS. Our study suggested that the LCQ-MC was well employed to assess cough after surgery and at the one-month follow-up in NSCLC patients after VATS. The LCQ-MC can provide important additional information concerning the impact of postoperative cough (22).
Our study has several limitations that should be considered. First, our study was limited by the number of patients and parameters, and there were a few published studies that could be consulted. Second, lack of preoperative cough symptom assessment, we assessed patient whether cough before surgery by asking case history. Third, our research did not examine a long follow-up period in postoperative cough patients. Additional anesthesia-related parameters, such as anesthetic drug and dose, should be included for further research.
In conclusion, female sex, the duration of anesthesia over 164 minutes, lower paratracheal node resection and subcarinal node resection were independent risk factors related to cough in NSCLC patients after VATS. In addition, the LCQ-MC performed satisfactorily in describing the longitudinal changes in cough symptoms. The mechanisms and characteristics of cough after pulmonary resection require further clarification.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The West China Hospital of Sichuan University in Sichuan, China, approved the study. All the participants provided written informed consent.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Sarna L, Evangelista L, Tashkin D, et al. Impact of respiratory symptoms and pulmonary function on quality of life of long-term survivors of non-small cell lung cancer. Chest 2004;125:439-45. [Crossref] [PubMed]
- Sawabata N, Maeda H, Takeda S, et al. Persistent cough following pulmonary resection: observational and empiric study of possible causes. Ann Thorac Surg 2005;79:289-93. [Crossref] [PubMed]
- Yang P, Cheville AL, Wampfler JA, et al. Quality of life and symptom burden among long-term lung cancer survivors. J Thorac Oncol 2012;7:64-70. [Crossref] [PubMed]
- Li WW, Lee TW, Yim AP. Quality of life after lung cancer resection. Thorac Surg Clin 2004;14:353-65. [Crossref] [PubMed]
- Sawada S, Suehisa H, Yamashita M. Inhalation of corticosteroid and beta-agonist for persistent cough following pulmonary resection. Gen Thorac Cardiovasc Surg 2012;60:285-8. [Crossref] [PubMed]
- Poghosyan H, Sheldon LK, Leveille SG, et al. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung Cancer 2013;81:11-26. [Crossref] [PubMed]
- Shure D. Endobronchial suture. A foreign body causing chronic cough. Chest 1991;100:1193-6. [Crossref] [PubMed]
- Karlsson JA. The role of capsaicin-sensitive C-fibre afferent nerves in the cough reflex. Pulm Pharmacol 1996;9:315-21. [Crossref] [PubMed]
- Sawabata N, Takeda S, Tokunaga T, et al. Acid regurgitation associated with persistent cough after pulmonary resection: an observational study. Cough 2006;2:9. [Crossref] [PubMed]
- Huang J, Luo Q, Tan Q, et al. Evaluation of the surgical fat-filling procedure in the treatment of refractory cough after systematic mediastinal lymphadenectomy in patients with right lung cancer. J Surg Res 2014;187:490-5. [Crossref] [PubMed]
- McKenna RJ Jr. New approaches to the minimally invasive treatment of lung cancer. Cancer J 2005;11:73-6. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- Park JS, Kim K, Choi MS, et al. Video-Assisted Thoracic Surgery (VATS) lobectomy for pathologic stage I non-small cell lung cancer: a comparative study with thoracotomy lobectomy. Korean J Thorac Cardiovasc Surg 2011;44:32-8. [Crossref] [PubMed]
- Nakazawa S, Shimizu K, Mogi A, et al. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg 2018;66:81-90. [Crossref] [PubMed]
- Liu L, Che G, Pu Q, et al. A new concept of endoscopic lung cancer resection: single-direction thoracoscopic lobectomy. Surg Oncol 2010;19:e71-7. [Crossref] [PubMed]
- Sihoe AD, Van Schil P. Non-small cell lung cancer: when to offer sublobar resection. Lung Cancer 2014;86:115-20. [Crossref] [PubMed]
- Zhi XY, Yu JM, Shi YK. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2015 version). Cancer 2015;121 Suppl 17:3165-81. [Crossref] [PubMed]
- Yang M, Fan J, Zhou H, et al. Zhongguo Fei Ai Za Zhi 2015;18:512-7. [What are the advantages? A prospective analysis of 16 versus 28 French chest tube sizes in video-assisted thoracoscopic surgery lobectomy of lung cancer]. [PubMed]
- Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58:339-43. [Crossref] [PubMed]
- Gao YH, Guan WJ, Xu G, et al. Validation of the Mandarin Chinese version of the Leicester Cough Questionnaire in bronchiectasis. Int J Tuberc Lung Dis 2014;18:1431-7. [Crossref] [PubMed]
- Lin R, Che G. Validation of the Mandarin Chinese version of the Leicester Cough Questionnaire in non-small cell lung cancer patients after surgery. Thorac Cancer 2018;9:486-90. [Crossref] [PubMed]
- Beaton DE, Boers M, Wells GA. Many faces of the minimal clinically important difference (MCID): a literature review and directions for future research. Curr Opin Rheumatol 2002;14:109-14. [Crossref] [PubMed]
- Raj AA, Pavord DI, Birring SS. Clinical cough IV: what is the minimal important difference for the Leicester Cough Questionnaire? Handb Exp Pharmacol 2009.311-20. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol 2015;10:1240-2. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The eighth edition lung cancer stage classification. Chest 2017;151:193-203.
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Kavalcikova-Bogdanova N, Buday T, Plevkova J, et al. Chronic cough as a female gender issue. Adv Exp Med Biol 2016;905:69-78. [Crossref] [PubMed]
- French CT, Fletcher KE, Irwin RS. A comparison of gender differences in health-related quality of life in acute and chronic coughers. Chest 2005;127:1991-8. [Crossref] [PubMed]
- Kvachadze I, Tsagareli MG, Dumbadze Z. An overview of ethnic and gender differences in pain sensation. Georgian Med News 2015.102-8. [PubMed]
- Dicpinigaitis PV, Rauf K. The influence of gender on cough reflex sensitivity. Chest 1998;113:1319-21. [Crossref] [PubMed]
- Song WJ, Chang YS. Cough hypersensitivity as a neuro-immune interaction. Clin Transl Allergy 2015;5:24. [Crossref] [PubMed]
- Morice AH, Jakes AD, Faruqi S, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J 2014;44:1149-55. [Crossref] [PubMed]
- Irwin RS. Complications of cough: ACCP evidence-based clinical practice guidelines. Chest 2006;129:54s-8s. [Crossref] [PubMed]
- Popat M, Mitchell V, Dravid R, et al. Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia 2012;67:318-40. [Crossref] [PubMed]
- Neelakanta G, Miller J. Minimum alveolar concentration of isoflurane for tracheal extubation in deeply anesthetized children. Anesthesiology 1994;80:811-3. [Crossref] [PubMed]
- Minogue SC, Ralph J, Lampa MJ. Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anesthesia. Anesth Analg 2004;99:1253-7. [Crossref] [PubMed]
- Sun L, Guo R, Sun L. The impact of prophylactic intravenous lidocaine on opioid-induced cough: a meta-analysis of randomized controlled trials. J Anesth 2014;28:325-33. [Crossref] [PubMed]
- Cho HB, Kim JY, Kim DH, et al. Comparison of the optimal effect-site concentrations of remifentanil for preventing cough during emergence from desflurane or sevoflurane anaesthesia. J Int Med Res 2012;40:174-83. [Crossref] [PubMed]
- Liu XS, Xu GH, Shen QY, et al. Dezocine prevents sufentanil-induced cough during general anesthesia induction: a randomized controlled trial. Pharmacol Rep 2015;67:52-5. [Crossref] [PubMed]
- Shuying L, Ping L, Juan N, et al. Different interventions in preventing opioid-induced cough: a meta-analysis. J Clin Anesth 2016;34:440-7. [Crossref] [PubMed]
- Arain SR, Shankar H, Ebert TJ. Desflurane enhances reactivity during the use of the laryngeal mask airway. Anesthesiology 2005;103:495-9. [Crossref] [PubMed]
- White PF, Tang J, Wender RH, et al. Desflurane versus sevoflurane for maintenance of outpatient anesthesia: the effect on early versus late recovery and perioperative coughing. Anesth Analg 2009;109:387-93. [Crossref] [PubMed]
- Polverino M, Polverino F, Fasolino M, et al. Anatomy and neuro-pathophysiology of the cough reflex arc. Multidiscip Respir Med 2012;7:5. [Crossref] [PubMed]
- Canning BJ, Chang AB, Bolser DC, et al. Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest 2014;146:1633-48. [Crossref] [PubMed]


